Summary of Clinical Trials for the Prevention and Treatment of Cardiomyopathy Related to Anthracyclines and HER2-Targeted Agents
Introduction
Due to great advancements in cancer treatments, patients with cancer are living longer, and the number of cancer survivors is increasing. As of January 2019, there were 16.9 million patients with a history of cancer in the United States. This number is expected to increase to 22.1 million by the year 2030.1 As life expectancy for patients with cancer improves, the cardiotoxic effects of chemotherapy are becoming an important determinant of long-term morbidity and mortality. Cardiotoxicity can both limit oncologic treatment and worsen long-term morbidity and mortality for these patients. Anthracyclines and anti-human epidermal growth factor receptor 2 (HER2) agents have classically been recognized as common causes of chemotherapy-related cardiac dysfunction. However, more recently, and with greater use of novel targeted agents, a heterogeneous spectrum of cardiotoxicities can occur due to modern cancer treatments. This review focuses on notable clinical trials that have investigated the effects of pharmacologic and invasive interventions in the prevention and treatment of cardiotoxicity manifest as heart failure (HF). The studies included here predominantly evaluated patients receiving anthracyclines and trastuzumab, agents that have well-established cardiotoxicity profiles.
Neurohormonal Therapy
HF following administration of anthracyclines and anti-HER2 agents such as trastuzumab has been the focus of many studies over the past two decades. The two classes of drugs have very different toxicity profiles, where anthracyclines typically cause irreversible myocardial damage and poor long-term outcomes, and trastuzumab effects are usually reversible with cessation of therapy. All of the studies reviewed here, except one,2 evaluate the effects of neurohormonal therapy when administered alongside chemotherapy.
Many trials have sought to investigate the effects of beta-blockers, angiotensin-converting enzyme inhibitors (ACEi), angiotensin-receptor blockers (ARB), or mineralocorticoid receptor antagonists; however, there are significant study limitations to be acknowledged. Most of the studies are small in number and include short length of follow-up. Many use imaging parameters or biomarkers as the primary endpoints rather than clinical outcomes. Comparisons between studies are limited due to different types of beta-blockers and ACEi/ARB and different cardiac endpoints utilized across studies.
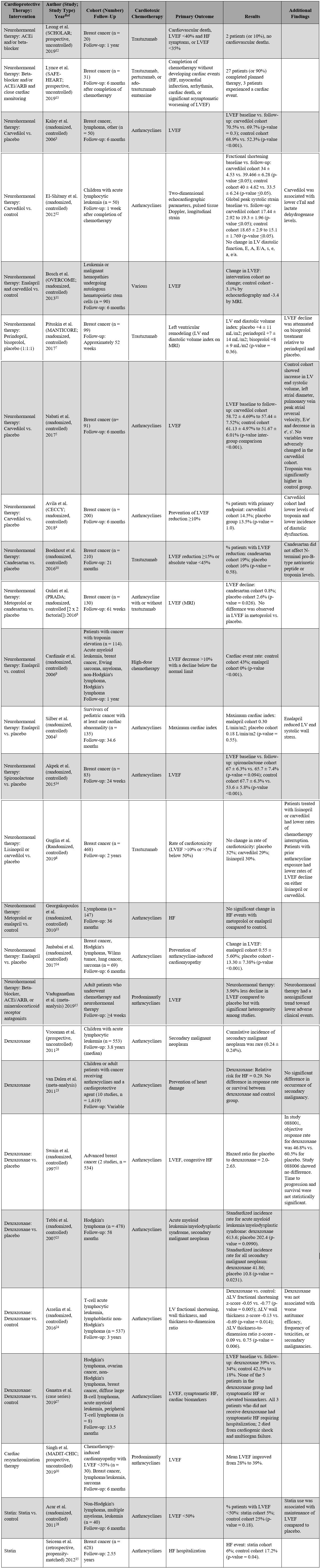
A list of the major clinical studies is detailed in Table 1. Below, notable findings are highlighted. Overall, the studies show a possible modest benefit from neurohormonal therapy in prevention of left ventricular ejection fraction (LVEF) reduction; however, the clinical significance remains unclear.
Also worth considering is whether a higher LVEF in the context of taking vasodilator therapy truly implies cardioprotection. For any given degree of inherent contractility, patients treated with vasodilators such as ACEi/ARB will have a higher LVEF. Therefore, it is unclear if a mildly higher LVEF in a treatment arm with these agents means any cardioprotection has occurred, as opposed to the expected small LVEF rise seen with active vasodilator therapy.
Table 1: List of Major Clinical Trials for the Prevention and Treatment of Cardiotoxicity Related to Anthracyclines and HER2-Targeted Agents
Beta-Blockers
Kalay et al.3 showed that in patients with cancer who are receiving anthracycline agents, carvedilol administration prevented decline in LVEF compared to the control cohort (carvedilol baseline ejection fraction of 70.5% to follow-up of 69.7% vs. control baseline ejection fraction of 68.9% to follow-up of 52.3%). Notably, doxorubicin doses listed in Table 1 (514-525 mg/m2 over 6 cycles) were inconsistent with typical dosing strategies, and carvedilol was dosed once daily despite typically being a twice-daily medication. Patients with congestive HF, cardiomyopathy, coronary artery disease, or moderate to severe mitral or aortic valve disease were excluded. These results were not reproduced in the subsequent CECCY (Carvedilol Effect in Preventing Chemotherapy Induced Cardiotoxicity)4 trial. The CECCY trial randomized 200 patients with HER-2-negative breast cancer who were referred to receive typical-dose anthracyclines (doxorubicin 240 mg/m2) to either carvedilol therapy or placebo. At a follow-up of 6 months, there was no difference in the primary endpoint of significant LVEF decline between the groups (carvedilol cohort = 14.5% vs. placebo = 13.5%; p-value = 1.0); however, the authors did report lower troponin levels in the carvedilol cohort and a lower incidence of diastolic dysfunction. The attenuation of troponin levels with carvedilol has been reported previously,5 although the mechanism of this effect and whether decreasing troponin levels improves outcomes remains unclear. The presence of baseline cardiovascular risk factors was low in the intervention group (hypertension = 3.1%, diabetes mellitus = 4.1%, hypercholesterolemia under statin treatment = 6.2%, current/past smoker = 25%). The patients included in both the CECCY trial and the trial by Kalay et al. represent a younger population at lower cardiovascular risk; therefore, these results may not be generalizable to high-risk patients.
The PRADA (Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy) trial was a 2 x 2 factorial, randomized controlled trial of candesartan and metoprolol in women with early breast cancer undergoing therapy with anthracyclines with or without trastuzumab.6 Metoprolol use was not associated with attenuation in LVEF reduction. Notably, LVEF was measured by magnetic resonance imaging (MRI), and measurements were made by echocardiography in the CECCY trial.
The MANTICORE (Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research) trial7 investigated the effects of beta-blockers in trastuzumab-related cardiotoxicity; 99 patients with breast cancer who received trastuzumab were randomized to receive perindopril, bisoprolol, or placebo (1:1:1). The majority of these patients (77%) was anthracycline-naïve. There was no significant difference between groups in the primary endpoint of left ventricular (LV) remodeling defined as a change in the indexed LV end diastolic volume on cardiac MRI. However, reduction in LVEF was attenuated in patients receiving bisoprolol compared to perindopril and placebo (bisoprolol = -1 ± 5% vs. perindopril = -3 ± 4% vs. placebo = -5 ± 5%; group p-value = 0.001). Cardioprotective agents were generally well tolerated in this study and overall decreased the incidence of interruption of chemotherapy. The rate of therapy interruption was 9% (3/33) of patients for perindopril, 10% (3/31) of patients for bisoprolol, and 30% (9/30) of patients in the placebo group (p-value = 0.03).
Guglin et al.8 randomized 468 women with HER2+ breast cancer on trastuzumab to treatment with lisinopril, carvedilol, or placebo. There was no significant difference in the primary endpoint of rates of cardiotoxicity (32% on placebo, 29% on carvedilol, 30% on lisinopril). Though not the primary objective of the study, they did find that patients who were treated with either lisinopril or carvedilol had lower rates of chemotherapy interruption. In addition, the subgroup of patients who had also previously been treated with anthracyclines had lower rates of LVEF reduction on either lisinopril or carvedilol when compared to placebo (carvedilol = -4.5 ± 0.8%, lisinopril = -4.5 ± 0.8%, placebo = -7.7 ± 0.8%). This effect was not seen in patients on trastuzumab who had not been treated with anthracyclines. The study was not powered to detect a difference in efficacy between lisinopril and carvedilol.
ACEi/ARB
Enalapril and candesartan have been studied against placebo in the prevention of chemotherapy-related cardiomyopathy. In survivors of pediatric cancer diagnosed with a cardiac abnormality, enalapril appeared to decrease the LV end-systolic wall stress, although it did not improve maximal cardiac index on exercise echocardiography testing.2 The benefits of enalapril were also evaluated by Cardinale et al.9 in patients with various cancer types who had troponin elevations shortly following high-dose chemotherapy (multiple regimens were included, although all patients had either prior or current anthracycline exposure). Patients who were randomized to receive enalapril following an abnormal troponin level showed a 0% rate of cardiac events compared to 43% in the control group (p-value <0.001).9 Cardiac events were defined as a reduction in LVEF >10% and below the normal limit. In contrast, candesartan was not associated with improved cardiac outcomes in patients with breast cancer on trastuzumab therapy (candesartan cardiac events rate = 19% vs. placebo = 16%; p-value = 0.58)10 in a study by a different group, in a cohort of patients who had all previously received anthracyclines.
Although the PRADA trial6 did not report a difference in LVEF in patients treated with metoprolol versus control, it did show a statistically significant attenuation of LVEF decline in patients treated with candesartan compared to placebo. LVEF declined 0.8% compared to 2.6% in the placebo group (p-value = 0.026); however, the clinical significance of this small absolute difference in LVEF is questionable.
Combination Therapy (Beta-Blockers and/or ACEi/ARB)
The OVERCOME (Prevention of Left Ventricular Dysfunction with Enalapril and Carvedilol in Patients Submitted to Intensive Chemotherapy for the Treatment of Malignant Hemopathies) trial11 was a randomized, controlled study that investigated the effect of treatment with enalapril and carvedilol in preventing changes in LVEF in 90 patients with leukemia or malignant hemopathies undergoing autologous bone marrow transplant. The majority of these patients received anthracyclines as part of their oncologic treatment (82% in the intervention group and 78% in the control group). Combined therapy with cardioprotective agents prevented LVEF reduction compared to the control group at a 6-month follow-up (-3.1% absolute difference by echocardiography; p-value = 0.035). There was no difference in total mortality between the groups; however, the intervention group had a lower incidence of a combined event of death or HF (6.7% vs. 22%; p-value = 0.036). There was no difference in serious adverse events between groups.
Combination therapy with ACEi/ARB and/or beta-blockers may be used to manage mild trastuzumab-related cardiomyopathy to enable completion of trastuzumab therapy. The SCHOLAR (Safety of Continuing Chemotherapy in Overt Left Ventricular Dysfunction Using Antibodies to HER-2) trial evaluated cardiac events in 20 patients with breast cancer on trastuzumab therapy who received ACEi and/or beta-blockers. Notably, all patients included in this study received doxorubicin with a median time between the last dose of doxorubicin to initiation of trastuzumab of 34 days. With this approach, 2 patients (10%) experienced a cardiac event defined as an LVEF reduction to less than 40% with HF symptoms.12 In the prospective SAFE-HEART (Cardiac Safety Study in Patients With HER2 + Breast Cancer) study of 31 patients with breast cancer, 90% of patients were able to safely complete HER2-targeted therapy with cardioprotective medications and close monitoring.13 Although these data provide some reassurance that mild cardiomyopathy should not necessarily preclude completion of trastuzumab, larger randomized studies are needed to further study the risks.
Mineralocorticoid Receptor Antagonists
The use of mineralocorticoid receptor antagonists has not been well studied in the cardio-oncology population. One study of 83 patients with breast cancer receiving anthracyclines showed that spironolactone therapy attenuated LVEF decline at 24-week follow-up. The spironolactone cohort changed from 67% to 65.7% versus the change in the control group of 67.7% to 53.6%.14
Meta-Analysis
A recent meta-analysis by Vaduganathan et al.15 included 1,984 patients across 17 randomized controlled trials of adult patients who had chemotherapy and neurohormonal therapy. These patients were on beta-blockers, mineralocorticoid receptor antagonists, or ACEi/ARB. Most of the included studies were of young patients (age range 38.7-52.9 years), and only a minority of patients in these studies had cardiovascular comorbidities such as hypertension or diabetes. Patients treated with neurohormonal therapy had a small but statistically significant attenuation of LVEF reduction (by 3.96%); however, significant heterogeneity among studies limits the interpretation of these results. Unfortunately, the effect of neurohormonal therapy on the long-term clinical outcomes of cardio-oncology patients remains unknown.
Markers of Subclinical Cardiac Dysfunction
Abnormal cardiac biomarkers and global longitudinal strain may be used to screen for subclinical cardiac dysfunction in patients receiving potentially cardiotoxic medications.16 Cardiac troponin I (cTnI) is a sensitive and specific marker of myocardial injury, and troponin elevation during chemotherapy administration has been associated with subsequent cardiac events. In a study by Cardinale et al.,17 703 patients with cancer receiving high-dose chemotherapy were divided into 3 groups based on troponin levels measured early and late into treatment: negative early and late cTnI levels, positive early cTnI and negative late cTnI levels, positive early and late cTnI levels. Patients with negative troponins at both time points had no significant reduction in LVEF during follow-up. Patients with positive troponins had the following cardiac event rate: 37% in the positive early cTnI and negative late cTnI group compared to 84% in the positive early and late cTnI group (p-value <0.001). Importantly, these dramatic results have yet to be replicated by other investigators, and the protocol required very frequent troponin measurements after chemotherapy administration. There are no established guidelines regarding the optimal frequency of troponin measurements during chemotherapy or a recommended threshold of troponin elevation that would warrant initiation of cardioprotective medications; however, elevated troponin values can be useful in defining patients at high risk for developing cardiotoxicity who may benefit from a cardiology consultation, closer monitoring, and cardiovascular risk factor management.
Global longitudinal strain is a measure of myocardial deformation that can detect early changes in cardiac function prior to LVEF reduction. The American Society of Echocardiography and the European Association of Cardiovascular Imaging recommend cardiology consultation for patients with baseline abnormal global longitudinal strain or positive troponins prior to initiation of chemotherapy associated with irreversible cardiac dysfunction.16 Negishi et al.18 measured global longitudinal strain in 81 women receiving trastuzumab, 37 of whom also received anthracyclines concurrently, and found that global longitudinal strain was a strong predictor of later cardiotoxicity. A reduction in global longitudinal strain of 11% with a 95% confidence interval of 8.3-14.6% had a sensitivity of 65% and specificity of 94% for prediction of chemotherapy-related cardiotoxicity. A worsening in global longitudinal strain of greater than 15% after initiation of chemotherapy was proposed as significant and a sign of subclinical LV dysfunction. However, 6-month LVEF drop appeared to be similarly predictive as 6-month global longitudinal strain change of 12-month LVEF drop, and the study did not include a validation cohort. In addition, many factors can affect strain independently of any true change in myocardial function, and measurements can vary significantly on echocardiography machines made by different vendors.19 Another study of 81 patients planning to undergo anthracycline and trastuzumab treatment reported the ability of post-anthracycline global longitudinal strain to predict subsequent LVEF drops with trastuzumab.20 However, it was once again unclear if this was better than LVEF as a predictor, particularly given that the authors excluded patients with post-anthracycline LVEF reduction from the analysis. Importantly, the definitions of chemotherapy-related cardiotoxicity have been variable and of unclear clinical significance, and none of the studies have prospectively tested a given global longitudinal strain cutoff value as significant or included a validation cohort. Although some have advocated initiating neurohormonal therapy when a global longitudinal strain change is observed, data regarding the benefit of such an approach are limited.16 A recent manuscript from Oiknomou et al.21 noted that it is unclear if the proposed relative global longitudinal strain cutoff of 15% is a sensitive or specific threshold and highlighted the need for external validation of the recommended cutoff values in different centers and vendors. The manuscript further noted a high risk of bias and confounders in almost all the published global longitudinal strain studies. For all of these reasons, we would recommend great caution in making clinical decisions based on global longitudinal strain changes alone until a clearer evidence base is established.
Dexrazoxane
Dexrazoxane is an intracellular chelating agent that is believed to interfere with iron-mediated oxygen free radical generation and inhibit topoisomerase II, thereby exerting a cardioprotective effect in the setting of anthracycline administration. In a paper by Swain et al.,22 the authors reported that dexrazoxane significantly reduces the incidence of LVEF decline and congestive HF based on the results from 2 multicenter, double-blind studies. The hazard ratio of placebo compared to dexrazoxane was 2.0 and 2.63 for the 2 studies. However, concerns over the possible concomitant reduction of the antineoplastic effect of anthracyclines prevented widespread use of dexrazoxane. One of the 2 studies included in the authors' analysis showed a decrease in response rates for dexrazoxane (46.8%) versus placebo (60.5%), although progression and survival were not statistically significant. A subsequent meta-analysis of 10 studies23 confirmed a cardioprotective effect of dexrazoxane (relative risk for HF was 0.29 with dexrazoxane use) and showed no difference in response rates, survival, or occurrence of secondary malignancy. Their findings were confirmed in a recent randomized controlled study by Asselin et al.,24 which showed that among 537 children and adolescents with T-cell acute lymphoblastic leukemia or lymphoblastic non-Hodgkin lymphoma receiving doxorubicin with or without dexrazoxane, administration of dexrazoxane was associated with improved LV fractional shortening, LV wall thickness, and thickness-to-ratio dimension, with no association with worse antitumor efficacy or an increase in toxicities or secondary malignancies.
The possibility that dexrazoxane increases the rate of secondary malignancies was raised after a 2007 study by Tebbi et al.25 showed that the standardized incidence rate for all secondary malignant neoplasms was 41.86 times greater than that of the general population for patients treated with dexrazoxane, and it was 10.8 for those treated without dexrazoxane. Subsequently, Vrooman et al.26 found that the 5-year incidence of secondary malignancies was very rare in 553 children with acute lymphocytic leukemia (0.24 ± 0.24%). Dexrazoxane is currently approved by the US Food and Drug Administration only for the treatment of advanced breast cancer in patients who have already received a cumulative dose of doxorubicin ≥300 mg/m2.
Patients with pre-existing cardiomyopathy have a high risk for cardiotoxicity, and anthracycline use in this patient population has generally been limited. Dexrazoxane has been used off-label as a cardioprotectant in this setting with some promising results, although large prospective studies are still needed. In a consecutive case series study, Ganatra et al.27 found that 5 patients with asymptomatic reduction in LVEF who received anthracycline with concomitant off-label dexrazoxane had minimal LVEF change following therapy (39% at baseline to 34% after chemotherapy). All 5 patients received doxorubicin at a dose of 280-300 mg/m2. In comparison, 3 patients treated with anthracyclines (doxorubicin 300 mg/m2, doxorubicin 120 mg/m2, and daunorubicin 540 mg/m2, respectively) without dexrazoxane had a marked reduction in LVEF (42.5% at baseline to 18% after chemotherapy).
Statin Therapy
Few studies have evaluated the effect of statin use for the prevention of cardiotoxicity. A study by Acar et al.28 randomized patients with non-Hodgkin's lymphoma, multiple myeloma, or leukemia to atorvastatin use or control and found that atorvastatin therapy was associated with prevention of LVEF decline (statin cohort = 1.3 ± 3.8 vs. control -7.9 ± 8.0; p-value <0.001). The mechanism of this effect is unknown but has been hypothesized to be due to the antioxidant and anti-inflammatory effect of statins. However, widespread evidence for statins for cardioprotection in cancer therapy remains lacking. The effect of atorvastatin on LVEF at 24 months after adjuvant anthracycline-based chemotherapy initiation in patients with breast cancer is currently being investigated in the National Institutes of Health (NIH) sponsored study PREVENT (Preventing Anthracycline Cardiovascular Toxicity with Statins).29
Cardiac Resynchronization Therapy
MADIT-CHIC (Multicenter Automatic Defibrillator Implantation Trial–Chemotherapy-Induced Cardiomyopathy) is the first study to evaluate the efficacy of chronic resynchronization therapy in chemotherapy-related cardiomyopathy.30 In a prospective, uncontrolled study, 30 patients with chemotherapy-induced cardiomyopathy (predominantly related to anthracyclines) with LVEF <35% who met American College of Cardiology, American Heart Association, and Heart Rhythm Society guideline indications for rhythm device therapy underwent chronic resynchronization therapy placement. At a follow-up of 6 months, LVEF improved from 28% to 39% (difference of 10.6%; p-value <0.001). These effects were similar to those previously reported in MADIT-CRT for patients with nonischemic cardiomyopathy.30
Conclusion
Neurohormonal therapy may attenuate LVEF reduction in patients receiving cardiotoxic chemotherapy; however, these changes appear to be small on short-term follow-up with unclear clinical significance. Carvedilol has been the best studied, particularly in patients exposed to anthracycline therapy. Metoprolol does not seem to be cardioprotective in patients on anthracyclines with or without trastuzumab. Many ACEi/ARB agents have been studied, including enalapril, candesartan, and lisinopril; most of these studies suggest a small attenuation of LVEF reduction. There are not enough data to support the use of one agent over another. Cardioprotective medications appear to be generally well tolerated in the cardio-oncology patient population, and they may decrease chemotherapy interruption, particularly for patients who are receiving trastuzumab. Overall, studies of neurohormonal antagonists for cardioprotection from anthracyclines and/or trastuzumab struggle from methodologic challenges, small sample size, heterogeneity among studies/patient populations, and, in most cases, small—if any—significant treatment effect. Abnormal troponin values predict later development of cardiotoxicity; however, there are limited data on the long-term benefits of initiation of cardioprotective agents for subclinical LV dysfunction. These patients are at high risk for deterioration to clinical LV dysfunction and would likely benefit from closer monitoring and cardiovascular risk factor reduction. Global longitudinal strain may be predictive for future reduction in LVEF, but the studies are small and suffer from significant methodologic flaws; it is unclear at this time what population—if any—would benefit from routine monitoring of global longitudinal strain.
There is good evidence that dexrazoxane is effective in preventing cardiotoxicity; however, it is not yet clear which patient-specific risk factors and conditions warrant initiation of cardioprotection with dexrazoxane. Early studies suggested that dexrazoxane might decrease oncologic response rates and increase the rate of secondary malignancies; however, subsequent long-term data do not support these early findings.
Cardiac resynchronization therapy may be beneficial in improving LVEF in selected patients with chemotherapy-induced cardiomyopathy who meet evidence-based indications on short-term follow-up. Long-term data with clinical outcomes are needed. The effects of mineralocorticoid receptor antagonists and statins in the prevention and treatment of cardiotoxicity need further study.
Future Avenues
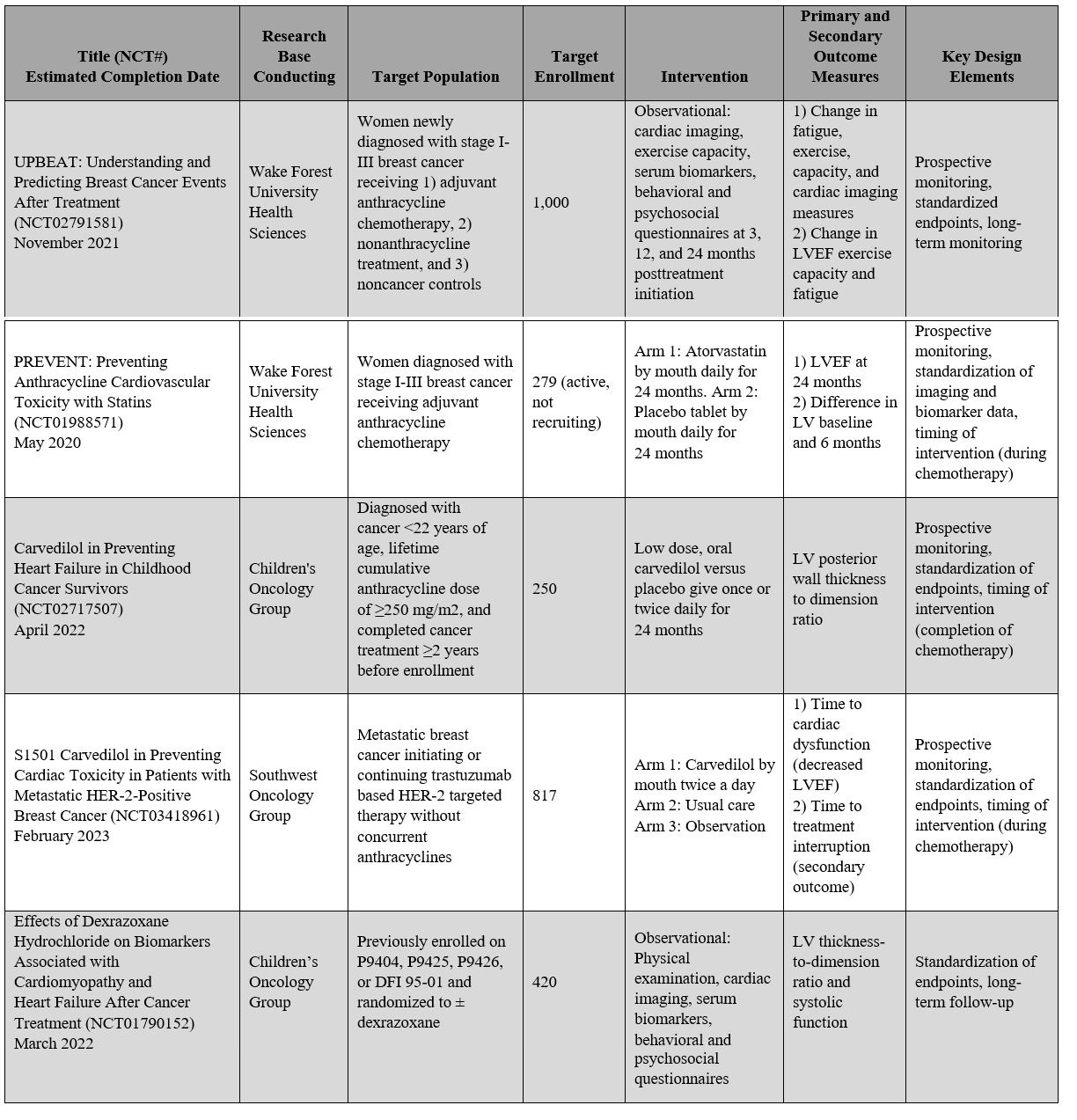
With increasing recognition and concern regarding chemotherapy-related cardiac morbidity and mortality, there is also increasing collaboration between oncologists and cardiologists to improve clinical trial study designs, which will enable a better understanding of cardiovascular toxicity of oncologic treatment. A standardized definition of cardiovascular toxicity that reflects clinically meaningful endpoints is needed to enable comparisons of data across studies. In addition, expanding study inclusion criteria to include patients who are at elevated cardiovascular risk is important because these patients may be most likely to benefit from cardioprotective medications and close monitoring by a cardio-oncologist. At this time, five major NIH-sponsored, oncology-based trials with integrated cardiovascular outcomes are now underway, a feature that has not previously been included in conventional oncology clinical trials (Table 2).31 Hopefully, new clinical trial designs that allow for a complete assessment of both cancer outcomes and cardiovascular toxicity will enable us to improve and optimize the care of all patients with cancer as well as cancer survivors.
Table 2: Active NIH-Sponsored Cardiovascular Toxicity Studies
References
- Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019;69:363-85.
- Silber JH, Cnaan A, Clark BJ, et al. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol 2004;22:820-8.
- Kalay N, Basar E, Ozdogru I, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol 2006;48:2258-62.
- Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR Jr, et al. Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity: The CECCY Trial. J Am Coll Cardiol 2018;71:2281-90.
- Nabati M, Janbabai G, Baghyari S, Esmaili K, Yazdani J. Cardioprotective Effects of Carvedilol in Inhibiting Doxorubicin-induced Cardiotoxicity. J Cardiovasc Pharmacol 2017;69:279-85.
- Gulati G, Heck SL, Ree AH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 × 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Hear J 2016;37:1671-80.
- Pituskin E, Mackey JR, Koshman S, et al. Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research (MANTICORE 101-Breast): A Randomized Trial for the Prevention of Trastuzumab-Associated Cardiotoxicity. J Clin Oncol 2017;35:870-7.
- Guglin M, Krischer J, Tamura R, et al. Randomized Trial of Lisinopril Versus Carvedilol to Prevent Trastuzumab Cardiotoxicity in Patients With Breast Cancer. J Am Coll Cardiol 2019;73:2859-68.
- Cardinale D, Colombo A, Sandri MT, e al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation 2006;114:2474-81.
- Boekhout AH, Gietema JA, Milojkovic Kerklaan B, et al. Angiotensin II-Receptor Inhibition With Candesartan to Prevent Trastuzumab-Related Cardiotoxic Effects in Patients With Early Breast Cancer: A Randomized Clinical Trial. JAMA Oncol 2016;2:1030-7.
- Bosch X, Rovira M, Sitges M, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol 2013;61:2355-62.
- Leong DP, Cosman T, Alhussein MM, et al. Safety of Continuing Trastuzumab Despite Mild Cardiotoxicity. JACC CardioOncol 2019;1:1-10.
- Lynce F, Barac A, Geng X, et al. Prospective evaluation of the cardiac safety of HER2-targeted therapies in patients with HER2-positive breast cancer and compromised heart function: the SAFE-HEaRt study. Breast Cancer Res Treat 2019;175:595-603.
- Akpek M, Ozdogru I, Sahin O, et al. Protective effects of spironolactone against anthracycline-induced cardiomyopathy. Eur J Hear Fail 2015;17:81-9.
- Vaduganathan M, Hirji SA, Qamar A, et al. Efficacy of Neurohormonal Therapies in Preventing Cardiotoxicity in Patients With Cancer Undergoing Chemotherapy. JACC CardioOncol 2019;1:54-65.
- Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014;27:911-39.
- Cardinale D, Sandri MT, Colombo A, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 2004;109:2749-54.
- Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr 2013;26:493-8.
- Voigt JU, Cvijic M. 2- and 3-Dimensional Myocardial Strain in Cardiac Health and Disease. JACC Cardiovasc Imaging 2019;12:1849-63.
- Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging 2012;5:596-603.
- Oikonomou EK, Kokkinidis DG, Kampaktsis PN, et al. Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. JAMA Cardiol 2019;4:1007-18.
- Swain SM, Whaley FS, Gerber MC, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol 1997;15:1318-32.
- van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev 2011;2011:CD003917.
- Asselin BL, Devidas M, Chen L, et al. Cardioprotection and Safety of Dexrazoxane in Patients Treated for Newly Diagnosed T-Cell Acute Lymphoblastic Leukemia or Advanced-Stage Lymphoblastic Non-Hodgkin Lymphoma: A Report of the Children's Oncology Group Randomized Trial Pediatric Oncology Group 9404. J Clin Oncol 2016;34:854-62.
- Tebbi CK, London WB, Friedman D, et al. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol 2007;25:493-500.
- Vrooman LM, Neuberg DS, Stevenson KE, et al. The low incidence of secondary acute myelogenous leukaemia in children and adolescents treated with dexrazoxane for acute lymphoblastic leukaemia: a report from the Dana-Farber Cancer Institute ALL Consortium. Eur J Cancer 2011;47:1373-9.
- Ganatra S, Nohria A, Shah S, et al. Upfront dexrazoxane for the reduction of anthracycline-induced cardiotoxicity in adults with preexisting cardiomyopathy and cancer: a consecutive case series. Cardiooncology. 2019;5:1.
- Acar Z, Kale A, Turgut M, et al. Efficiency of atorvastatin in the protection of anthracycline-induced cardiomyopathy. J Am Coll Cardiol 2011;58:988-9.
- Preventing Anthracycline Cardiovascular Toxicity With Statins (PREVENT) (ClinicalTrials.gov website). May 20, 2020. Available at https://clinicaltrials.gov/ct2/show/NCT01988571. Accessed March 31, 2020.
- Singh JP, Solomon SD, Fradley MG, et al. Association of Cardiac Resynchronization Therapy With Change in Left Ventricular Ejection Fraction in Patients With Chemotherapy-Induced Cardiomyopathy. JAMA 2019;322:1799-805.
- Minasian LM, Dimond E, Davis M, et al. The Evolving Design of NIH-Funded Cardio-Oncology Studies to Address Cancer Treatment-Related Cardiovascular Toxicity. JACC CardioOncol 2019;1:105-13.
- El-Shitany NA, Tolba OA, El-Shanshory MR, El-Hawary EE. Protective effect of carvedilol on adriamycin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. J Card Fail 2012;18:607-13.
- Georgakopoulos P, Roussou P, Matsakas E, et al. Cardioprotective effect of metoprolol and enalapril in doxorubicin-treated lymphoma patients: a prospective, parallel-group, randomized, controlled study with 36-month follow-up. Am J Hematol 2010;85:894-6.
- Janbabai G, Nabati M, Faghihinia M, Azizi S, Borhani S, Yazdani J. Effect of Enalapril on Preventing Anthracycline-Induced Cardiomyopathy. Cardiovasc Toxicol 2017;17:130-9.
- Seicean S, Seicean A, Plana JC, Budd GT, Marwick TH. Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: an observational clinical cohort study. J Am Coll Cardiol 2012;60:2384-90.
Clinical Topics: Arrhythmias and Clinical EP, Cardio-Oncology, Dyslipidemia, Heart Failure and Cardiomyopathies, Noninvasive Imaging, Vascular Medicine, Implantable Devices, SCD/Ventricular Arrhythmias, Nonstatins, Novel Agents, Statins, Echocardiography/Ultrasound
Keywords: Cardio-oncology, Cardiotoxicity, American Heart Association, Angiotensin-Converting Enzyme Inhibitors, Anthracyclines, Antioxidants, Bisoprolol, Breast Neoplasms, Cardiac Resynchronization Therapy, Cardiology, Cardiomyopathies, Cardiotonic Agents, Chemotherapy, Adjuvant, Cohort Studies, Confidence Intervals, Control Groups, Daunorubicin, Defibrillators, Implantable, Dexrazoxane, DNA Topoisomerases, Type II, Double-Blind Method, Doxorubicin, Echocardiography, Enalapril, Follow-Up Studies, Heart Diseases, Hydroxymethylglutaryl-CoA Reductase Inhibitors, Incidence, Leukemia, Lisinopril, Metoprolol, Mineralocorticoid Receptor Antagonists, Multiple Myeloma, Off-Label Use, Perindopril, Referral and Consultation, Prospective Studies, Risk, Sensitivity and Specificity, Spironolactone, Stroke Volume, Troponin, Troponin I, United States Food and Drug Administration
< Back to Listings