Microvascular Thrombi in COVID-19
Quick Takes
- Coagulopathy in patients with severe coronavirus disease 2019 (COVID-19) is associated with increased mortality and characterized by increased D-dimer, increased lactate dehydrogenase, mild thrombocytopenia, elevated fibrinogen, and elevated von Willebrand factor (VWF).
- Diffuse pulmonary microvascular thrombi have been observed in autopsy studies of patients with COVID-19, with nine times the prevalence seen in acute respiratory distress syndrome (ARDS) due to influenza.
- Aberrant activation of immunothrombosis can trigger pathologic microvascular thrombi, and further research into these processes may result in new approaches to treat COVID-19.
Novel COVID-19 infection, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is dominated by respiratory tract infection. Yet a proportion of patients develop a more severe and systemic disease that is characterized by ARDS, shock, and multi-organ dysfunction. Microvascular thrombi are increasingly recognized as a significant contributor to the morbidity and mortality of COVID-19.1 Microvascular thrombi are hypothesized to explain the observation of profound hypoxia seen in some patients with COVID-19 with well-preserved lung mechanics. Macrovascular thrombotic complication incidence is high, reported up to 31% in patients with severe COVID-19,2,3 and includes venous thromboembolism (VTE), arterial thrombosis, and stroke. The incidence of microvascular thrombi is not known. In this analysis, we discuss the laboratory evidence for coagulopathy in COVID-19, the pathology evidence for microvascular thrombi in COVID-19, and the hypothesized mechanisms for microvascular thrombi initiation in response to COVID-19.
Evidence of COVID-19 Microvascular Thrombi
Microvascular thrombi in COVID-19 have been best described in autopsy studies with varying concordance. An Austrian case series of 11 patients who died of COVID-19 found thrombosis of small and mid-side pulmonary arteries of different degrees in all patients.4 A study of 10 patients who died of COVID-19 in New Orleans found thrombosis and microangiopathy in the small vessels and capillaries of the lungs.5 Yet an autopsy case series of patients with severe COVID-19 from Seattle found rare, focal pulmonary microthrombi in only 5 of 14 patients without evidence of endothelialitis.6 One autopsy study documented endothelialitis with virally infected endothelial cells,7 but this observation has been called into question.8 Another autopsy study compared lungs from patients with COVID-19, lungs from patients with ARDS due to H1N1, and uninfected control lungs.9 The investigators found that patients with COVID-19 had widespread thrombosis with microangiopathy, which was 9 times more prevalent than in patients who died from ARDS due to influenza. Importantly, microvascular thrombi were confirmed in an in vivo evaluation of sublingual microcirculation in 11 of 13 (85%) patients with severe COVID-19 requiring mechanical ventilation.10
More detailed studies explored the composition of the thrombi. A German case series of patients with COVID-19 found diffuse pulmonary microvascular thrombi that were characterized by aggregation of neutrophil extracellular traps.11 The most recent and largest autopsy study of 28 patients with COVID-19 revealed microvascular thrombi in the lungs, kidneys, and hearts.12 The investigators also found that these thrombi were characterized by neutrophil extracellular traps, associated with platelets and fibrin. In an autopsy study of 5 individuals who died of severe COVID-19 in New York, tissue damage consistent with complement-mediated microvascular injury was observed in the lungs and/or skin.13 In the lung pathology, significant capillary fibrin deposition and neutrophil permeation was seen with deposits of C5b-9 (membrane attack complex), C4d, and mannose-binding lectin-associated serine protease-2 in the microvasculature. These findings are consistent with sustained, systemic activation of the complement system.
Hypothesized Mechanisms of COVID-19 Microvascular Thrombi Initiation
The pathologic activation of the hemostatic process results in thrombosis. However, thrombosis also has a major physiological role in immune defense.14 Immunothrombosis is the activation of coagulation that accompanies host innate immune defense. In addition to the core hemostatic processes involving fibrin and platelets, thrombosis can be induced through cellular immune mediators such as neutrophils and molecular mediators such as intravascular tissue factors. Immunothrombosis most often occurs in the microvascular setting and contributes to pathogen recognition, best established against bacteria, and suppression of pathogen tissue invasion and dissemination. This process tends to occur in a restricted fashion in a small number of microvessels.
Uncontrolled activation or aberrant immunothrombosis can result in pathologic processes. These microvascular immunothrombi are commonly observed in ARDS but not to the degree seen in COVID-19. In addition, dysregulation of the complement pathway, a component of the innate immune system, is associated with the development of diffuse microvascular thrombi. These syndromes include catastrophic antiphospholipid antibody syndrome, atypical hemolytic uremic syndrome, purpura fulminans, and severe multiorgan malignant atrophic papulosis. Antiphospholipid antibodies have been described in patients with COVID-19.15
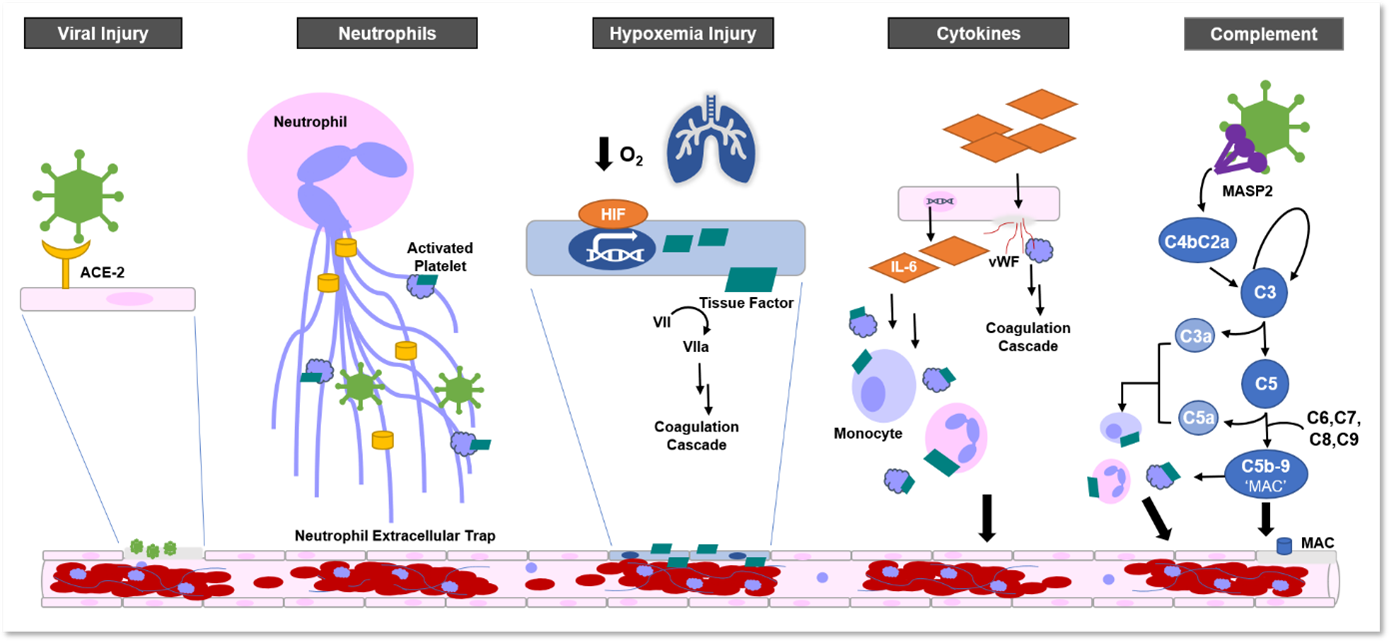
Immunothrombosis can be initiated by a number of pathways. These pathways have been hypothesized to be key players in the creation of diffuse microvascular thrombi in COVID-19. Some select examples are discussed in the following list (Figure 1):
- Direct viral infection. SARS-CoV-2 can directly infect tissue via the angiotensin-converting enzyme 2 receptor, which is found in the lungs, heart, kidneys, intestines, and endothelial cells. Viral infection can lead to endothelial injury that would initiate coagulation through classic hemostasis processes involving fibrin and platelets. There is mixed evidence supporting diffuse, direct invasion of the endothelium by SARS-CoV-2.6-8
- Neutrophils. Neutrophils clear viruses through phagocytosis of viral particles and by releasing neutrophil extracellular traps. Neutrophil extracellular traps are tangles of chromatin released from neutrophils with antimicrobial and nuclear proteins, used to contain infections. Neutrophil extracellular traps induce coagulation through tissue factor presentation, factor XII activation, and direct platelet binding and activation. Neutrophil extracellular traps are well-studied in other diseases, and intravascularly, neutrophil extracellular traps can contribute to occlusions of arteries, veins, and microvasculature. Myeloperoxidase-DNA complexes and citrullinated histone H3 are biomarkers used to measure levels of neutrophil extracellular traps in plasma, albeit with low specificity. Elevated levels of both biomarkers have been found in patients with severe COVID-19.16 COVID-19 autopsy studies also demonstrated evidence of neutrophil extracellular traps in microvascular thrombi.11-13
- Hypoxemia Injury. Hypoxemia is a hallmark of severe COVID-19 and can induce the expression of hypoxia-inducible transcription factors that upregulate tissue factor expression. Tissue factor is a transmembrane protein expressed by monocytes, vascular endothelial cells, and platelets. Tissue factor is a receptor and cofactor for coagulation factors VII and VIIa. Tissue factor promotes prothrombin to thrombin, which converts fibrinogen to fibrin, leading to fibrin-based clots. Both ARDS and microvascular thrombi will lead to hypoxemia, resulting in a thrombo-inflammatory feedback loop.17
- Cytokines. Cytokines can disrupt endothelial function and integrity, leading to release of VWF and additional pro-inflammatory cytokines that support the recruitment of platelets and leukocytes. In addition, pro-inflammatory cytokines, such as interleukin-6, can also promote tissue factor expression in monocytes, endothelium, and platelets, leading to coagulation.
- Complement cascade. The complement cascade can be initiated through the classical pathway, triggered by antibody-antigen complexes, the alternative pathway, stimulation by surface antigens, and the lectin pathway activated by binding mannose residues on the pathogen surface. These pathways converge on the common pathway, which terminates in the membrane attack complex (C5b-9). Multiple parts of the complement cascade can promote coagulation. Anaphylatoxins C5a and C3a facilitate the recruitment and activation of platelets, monocytes, and neutrophils, promoting the expression of tissue factor. Membrane attack complex also can activate platelets, injure microvascular endothelium, and subsequently activate the clotting pathway. MASP-2, a key complex in the lectin pathway, has been observed in COVID-19 vasculature.13 Vascular deposition of membrane attack complex is a key feature of many microthrombotic syndromes and has also been demonstrated in COVID-19.13
- Clinical trials directed at the complement cascade in the treatment of COVID-19 are underway: C1 esterase inhibitor ruconest (NCT04414631 and NCT04530136); C3 inhibitors AMY-101 (NCT04395456) and APL-9 (NCT04402060); C5 inhibitors eculizumab (NCT04346797 and NCT04355494), ravulizumab (NCT04570397, NCT04369469 and NCT04390464), and zilucoplan (NCT04382755); C5a inhibitor IFX-1 (NCT04333420); and C5a receptor inhibitor avdoralimab (NCT04371367).
Figure 1: Hypothesized Mechanisms of Microvascular Thrombosis in COVID-19
COVID-19 Coagulopathy
Coagulopathy in severe COVID-19 has been associated with high morbidity and mortality.18 COVID-19 coagulopathy is generally characterized by increased D-dimer (a fibrin-degradation product released when plasm cleaves cross-linked fibrin), increased lactate dehydrogenase, and a mild thrombocytopenia (100-150x109/L).19 In a study comparing non-survivors' and survivors' admission laboratory results, the investigators found a significant difference in the D-dimer (2.12 vs. 0.61 mcg/mL; p < 0.001), prothrombin time (PT) (15.5 vs. 13.6 sec; p < 0.001), and fibrin-degradation product (7.6 vs. 4.0 mcg/mL; p < 0.001).20 Activated partial thromboplastin time is typically normal. More in-depth studies of COVID-19 coagulopathy revealed elevated fibrinogen, factor VIII, and VWF.21,22 Patients with COVID-19 with VTE had higher D-dimer levels than patients without VTE.23
Coagulopathy in COVID-19 is unlike classic disseminated intravascular coagulopathy. Classic disseminated intravascular coagulopathy results in fulminant activation of coagulation, consumption of coagulation factors, and bleeding. Laboratory results reflect prolonged activated partial thromboplastin time and PT, thrombocytopenia (<50x109/L), moderately elevated D-dimer, and low fibrinogen (<1.0 g/L). Bleeding and microangiopathic thrombosis in multiple organs are characteristic of disseminated intravascular coagulopathy.24 Most patients with severe COVID-19 would not be classified as having disseminated intravascular coagulopathy according to the International Society on Thrombosis and Hemostasis because thrombocytopenia is mild, there is little change in PT, and excessive bleeding is rare. Disseminated intravascular coagulopathy consumptive coagulopathy is rarely seen in COVID-19. Only a small number of non-surviving patients with COVID-19 may meet the criteria for disseminated intravascular coagulopathy shortly prior to death.
Possible Treatment for Microvascular Thrombi
Currently, there are no evidence-based therapies for treating microvascular thrombi in COVID-19. Clinical trials are ongoing and target many of the proposed underlying mechanisms, including antiviral therapy, strategies for hypoxia management in COVID-19 ARDS, anti-cytokine therapies, and anti-complement cascade therapies.
Given the high risk of both macrovascular and microvascular thrombotic complications with COVID-19, empiric anticoagulation at prophylactic doses is frequently used as part of the standard of care for patients with COVID-19, but evidence-based benefits remain to be determined.1 Both the National Institutes of Health and the World Health Organization recommend that hospitalized adults with COVID-19 should receive prophylactic dose anticoagulation unless the risk of bleeding outweighs the risk of thrombosis. The American Society of Hematology recommends pharmacologic prophylaxis with low molecular weight heparin over unfractionated heparin to reduce contact. Extended post-discharge VTE prophylaxis can be considered in patients with limited mobility, but no studies specific to COVID-19 have been published.1 Empiric therapeutic dosing of anticoagulation is not currently recommended unless otherwise clinically indicated. However, a clinical trial investigating the safety and efficacy of apixaban in non-hospitalized adults with COVID-19 is underway (NCT04498273).
An early retrospective study from China demonstrated that patients with COVID-19 treated with prophylactic anticoagulation, primarily low molecular weight heparin, had a better prognosis with lower 28-day mortality than patients not on anticoagulation.25 The risk of bleeding on anticoagulation remains a concern. A single-center study of patients with COVID-19 demonstrated increased bleeding events and increased inpatient mortality associated with therapeutic, but not prophylactic, dosing.26 A propensity-matched cohort study found that patients with COVID-19 who had gastrointestinal bleeding during the hospitalization had increased mortality.28 Yet the use of anticoagulation or antiplatelet agents was not a risk factor for gastrointestinal bleeding. A larger study of 2,773 hospitalized patients with COVID-19 demonstrated that patients on anticoagulation had decreased in-hospital mortality and increased median survival (21 days compared to 14 days).27 The difference was more striking in patients with COVID-19 requiring mechanical ventilation; anticoagulation use was associated with decreased in-hospital mortality (29.1% vs. 62.7%) and longer median survival (21 days vs. 9 days).
Conclusions
Microvascular thrombi are commonly observed in patients with severe COVID-19 and contribute to significant morbidity and mortality. The aberrant immunothrombosis likely can be initiated by a number of pathways. More research is needed to elucidate these processes, and clinical trials are needed to evaluate whether targeting these pathways may ameliorate severe COVID-19.29
References
- Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol 2020;75:2950-73.
- Klok FA, Kruip MJ, van der Meer NJ, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145-7.
- Léonard-Lorant I, Delabranche X, Séverac F, et al. Acute Pulmonary Embolism in Patients with COVID-19 at CT Angiography and Relationship to d-Dimer Levels. Radiology 2020;296:E189-e191.
- Lax SF, Skok K, Zechner P, et al. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome : Results From a Prospective, Single-Center, Clinicopathologic Case Series. Ann Intern Med 2020;173:350-61.
- Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med 2020;8:681-6.
- Bradley BT, Maioli H, Johnston R, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet 2020;396:320-32.
- Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417-8.
- Goldsmith CS, Miller SE, Martines RB, Bullock HA, Zaki SR. Electron microscopy of SARS-CoV-2: a challenging task. Lancet 2020;395:e99.
- Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med 2020;383:120-8.
- do Espírito Santo DA, Lemos ACB, Miranda CH. In vivo demonstration of microvascular thrombosis in severe COVID-19. J Thromb Thrombolysis 2020;50:790-4.
- Leppkes M, Knopf J, Naschberger E, et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine 2020;58:102925.
- Nicolai L, Leunig A, Brambs S, et al. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy. Circulation 2020;142:1176-89.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res 2020;220:1-13.
- Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol 2013;13:34-45.
- Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med 2020;382:e38.
- Zuo Y, Yalavarthi S, Shi H, et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020;5:e138999.
- Yan SF, Mackman N, Kisiel W, Stern DM, Pinsky DJ. Hypoxia/Hypoxemia-Induced activation of the procoagulant pathways and the pathogenesis of ischemia-associated thrombosis. Arterioscler Thromb Vasc Biol 1999;19:2029-35.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-62.
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020;323:1061-9.
- Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844-7.
- Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 2020;18:1747-51.
- Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost 2020;18:1738-42.
- Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 2020;18:1421-4.
- Iba T, Levy JH, Warkentin TE, Thachil J, van der Poll T, Levi M. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost 2019;17:1989-94.
- Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020;18:1094-9.
- Musoke N, Lo KB, Albano J, et al. Anticoagulation and bleeding risk in patients with COVID-19. Thromb Res 2020;196:227-30.
- Paranjpe I, Fuster V, Lala A, et al. Association of Treatment Dose Anticoagulation With In-Hospital Survival Among Hospitalized Patients With COVID-19. J Am Coll Cardiol 2020;76:122-4.
- Trindade AJ, Izard S, Coppa K, et al. Gastrointestinal Bleeding in Hospitalized COVID-19 Patients: A Propensity Score Matched Cohort Study. J Intern Med 2020;Dec 20:[Epub ahead of print].
- Campbell CM, Kahwash R. Will Complement Inhibition Be the New Target in Treating COVID-19-Related Systemic Thrombosis? Circulation 2020;141:1739-41.
Clinical Topics: Anticoagulation Management, COVID-19 Hub, Diabetes and Cardiometabolic Disease, Dyslipidemia, Heart Failure and Cardiomyopathies, Prevention, Pulmonary Hypertension and Venous Thromboembolism, Vascular Medicine, Anticoagulation Management and Venothromboembolism, Lipid Metabolism, Novel Agents, Heart Failure and Cardiac Biomarkers, Pulmonary Hypertension, Hypertension
Keywords: Hypertension, Pulmonary, Histones, Peroxidase, Complement C1 Inhibitor Protein, Complement Membrane Attack Complex, COVID-19, Prothrombin, Thromboplastin, Thrombin, Neutrophils, Microcirculation, Mannose-Binding Protein-Associated Serine Proteases, Interleukin-6, von Willebrand Factor, Factor XII, Influenza A Virus, H1N1 Subtype, Mannose, Receptor, Anaphylatoxin C5a, Capillaries, Fibrin, Fibrinogen, Blood Platelets, Endothelial Cells, Chromatin, Antiphospholipid Syndrome, Hemostatics, Factor VIIa, Malignant Atrophic Papulosis, Pulmonary Artery, Purpura Fulminans, Nuclear Proteins, Cytokines, Venous Thromboembolism, Lectins, Monocytes, Antigens, Surface, Influenza, Human, Respiration, Artificial, Multiple Organ Failure, Respiratory Distress Syndrome, Hemostasis, Antibodies, Antiphospholipid, Stroke, Phagocytosis, Endothelium, Anti-Infective Agents, Biomarkers, Immunity, Innate, Virion, Mannose-Binding Lectins, Transcription Factors, Anaphylatoxins
< Back to Listings