Review of Late CV Effects After Hematopoietic Stem Cell Transplantation
Quick Takes
- Risk factors for cardiomyopathy in hematopoietic stem cell transplantation (HSCT) survivors include treatment-related effects such as anthracycline exposure and history of chest radiation, as well as conventional risk factors such as hypertension, diabetes, smoking, and ischemic heart disease.
- Cardiac injury occurs commonly to children undergoing HSCT, but the long-term implications of this are unknown.
- Collaborations between hematologists and cardiologists are crucial in limiting toxicity during HSCT and managing late complications.
Introduction
For several decades, HSCT has been used as curative treatment for many malignancies, hemoglobinopathies, metabolic diseases, bone marrow failure syndromes, and primary immune deficiencies. In brief, HSCT accomplishes disease eradication through high-dose chemotherapy with or without radiation, leading to ablation of a patient's bone marrow, known as conditioning, followed by transplantation of either donor-derived stem cells (allogeneic HSCT) or patient-derived stem cells collected prior to conditioning (autologous HSCT). HSCT allows for the delivery of cytotoxic therapies for the treatment of cancer that a patient would not otherwise tolerate due to prolonged myelosuppression.1,2 Allogeneic HSCT allows for replacement of defective host bone marrow stem cells, for example in the case of primary immune deficiency, and may derive benefits from the donor immune system developing a "graft-versus-tumor" effect that can eradicate underlying disease in the case of malignancy.3 Use of autologous transplants is more common in adults for the treatment of multiple myeloma and lymphoma, and allogeneic transplants are more common in pediatric patients for non-malignant conditions such as immune deficiencies, hemoglobinopathies, and metabolic diseases.1,4
Despite the improvements in survival afforded by HSCT,5 it is associated with significant short- and long-term morbidity and mortality.6 Almost all organ systems can be affected by the cytotoxic effects of the conditioning regimen, resulting cytopenias, and graft-versus-host disease, specifically in patients undergoing allogeneic HSCT. The direct toxic effects of HSCT include mucositis, bleeding, hepatic veno-occlusive disease, thrombotic microangiopathy, infections, delirium, malnutrition, interstitial pneumonia, acute kidney injury, and cardiovascular complications such as cardiomyopathy, arrhythmias, pulmonary hypertension, and pericardial effusions. The incidence of cardiovascular system complications is related to factors such as age at transplant, co-morbid medical conditions, use of cardiotoxic chemotherapy prior to HSCT, autologous versus allogeneic HSCT, and the conditioning regimen employed.6
Long-Term Cardiotoxicity After HSCT
Early reports of HSCT-related cardiotoxicity were published in 1976 and described 29 patients (17 pediatric) with leukemia, aplastic anemia, or metastatic cancer undergoing allogeneic HSCT.7 Post mortem studies revealed interstitial changes with infiltration of immune cells including macrophages and lymphocytes in 5 patients who demonstrated graft-versus-host disease. Extensive myocardial necrosis, fibrin deposits, and extravasation of red blood cells thought to be due to high-dose cyclophosphamide were seen in 2 patients who died from congestive heart failure.
Cardiovascular Disease in Adult Recipients
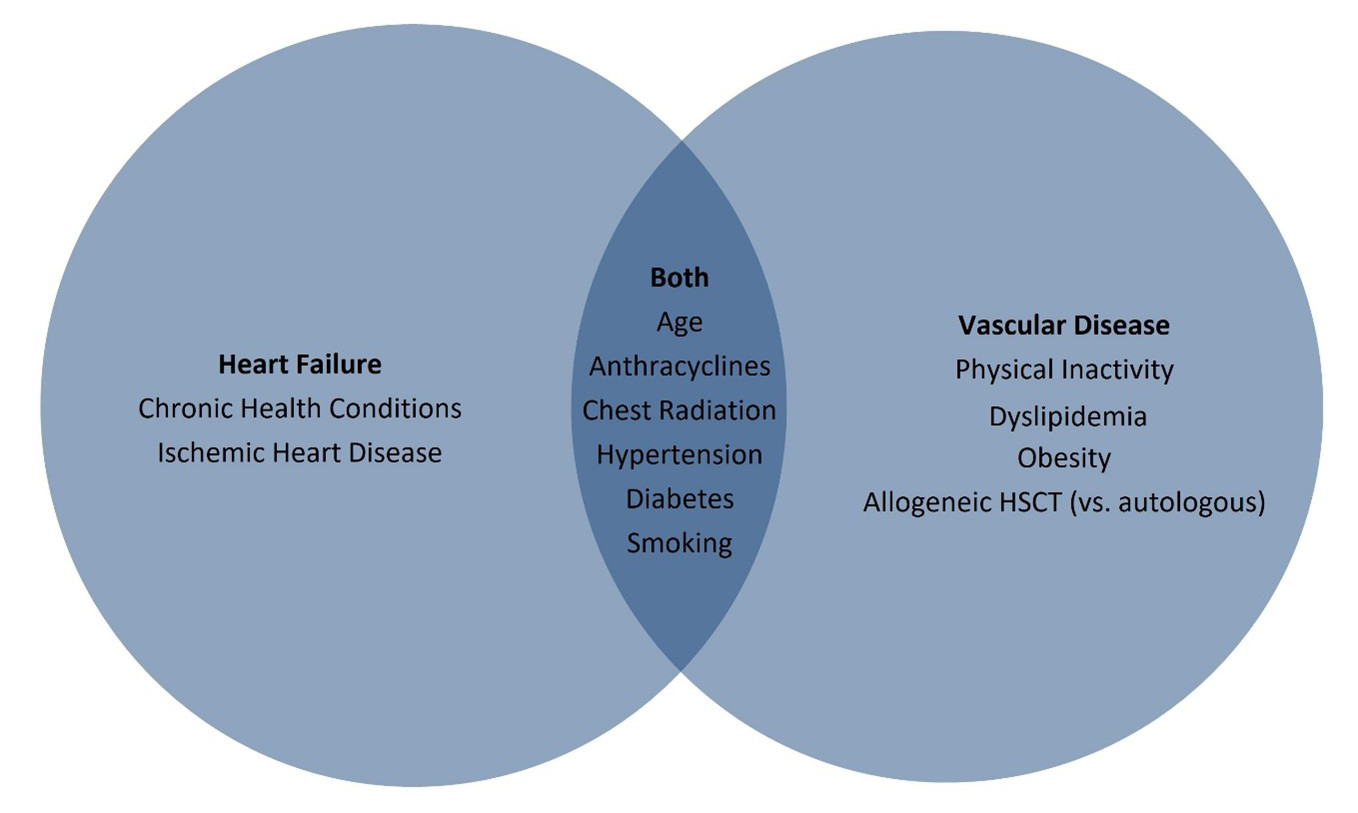
An observational study of 1,244 patients undergoing autologous HSCT (58% male, mean age of 44 years) reported 5-year incidence of congestive heart failure at 4.8%, rising to 9.1% at 15 years.8 Whether any of these events occurred in the period immediately surrounding HSCT or within the first 100 days post-transplant is not reported. Factors implicated in development of heart failure were older age, hypertension, diabetes mellitus, and prior exposure to anthracyclines at doses ≥250 mg/m2.8 Women were twice as likely as men to develop heart failure. These findings were consistent with an earlier case-control study that showed that pre-HSCT exposure to anthracyclines, number of chemotherapy cycles, and cardiovascular co-morbidities contributed to late-onset (>1 year) congestive heart failure but conditioning regimen and donor source (i.e., allogeneic vs. autologous) did not.9 Almost all (94%) cases of heart failure had reduced ejection fraction at the time of diagnosis. More data are available regarding long-term risks; anthracycline exposure ≥250 mg/m2, pre-existing hypertension, diabetes mellitus, and ischemic heart disease are risk factors for early- and late-onset cardiomyopathy post-HSCT (Figure 1).10,11
Figure 1: Risk Factors for Late CV Disease in HSCT Survivor
In addition to cardiomyopathy, other cardiovascular sequelae can occur in adult long-term survivors after HSCT.12 Compared with the general population, HSCT recipients demonstrate increased risk of cardiovascular death (adjusted incidence rate difference = 3.6 per 1,000 person years), which coincides with a higher incidence of ischemic heart disease, cardiomyopathy, stroke, and vascular disease.13 Risk factors for vascular events in HSCT survivors include allogeneic HSCT, hypertension, dyslipidemia, diabetes, radiation exposure, and increased rates of modifiable risk factors (obesity, smoking, and physical inactivity).10,12-15
An interesting phenomenon recently identified as a risk factor for cardiovascular disease after HSCT is clonal hematopoiesis of indeterminate potential.16,17 Clonal hematopoiesis is an expansion of blood cells originating from a single hematopoietic stem cell, which is associated with the development of myeloid neoplasms and cardiovascular disease in the general population.16,18 The mechanism through which clonal hematopoiesis of indeterminate potential is thought to increase the risk of cardiovascular disease is via an increase in systemic inflammation.16 In adults receiving autologous HSCT, the incidence of clonal hematopoiesis of indeterminate potential pre-HSCT increased with age, and clonal hematopoiesis of indeterminate potential was associated with an increased risk of death from cardiovascular disease.17 Similar results have not been seen in the allogeneic HSCT or pediatric HSCT setting.19,20
Cardiovascular Disease in Pediatric Recipients
Accurate determination of the incidence cardiovascular dysfunction in pediatric patients after HSCT is limited, and there is wide variation in the data available due to non-standardized definitions of cardiac dysfunction, variability in screening protocols, and recommended intervals for follow-up that may be months to decades. Among 661 children undergoing HSCT between 1995 and 2008 and surviving at least 2 years, coronary artery disease occurred in 0.2%, cerebrovascular accident in 0.6%, cardiomyopathy in 3%, and cardiac-related death in 0.5%; all are greater than the general population. Risk factors for adverse outcomes included anthracycline chemotherapy and history of cranial or chest radiation. Regarding risk factors for cardiovascular disease, dyslipidemia was prevalent in 18% and diabetes mellitus in 7% of patients.21 Another study of 162 children in the first 5 years post-HSCT reported a cumulative incidence of an abnormal left ventricular systolic function of 26%. As in adults, total body irradiation and previous anthracycline chemotherapy were strong risk factors for cardiac dysfunction.22 Similarly, in 95 patients at a mean of 13 years post-HSCT in the pediatric period, the negative predictive value of no exposure to total body irradiation or anthracyclines was 96.7% (95% confidence interval, 83.3-99.8%) for cardiac dysfunction.23 However, not all studies demonstrate a clear relationship between total body irradiation and cardiovascular disease. In a cohort of 826 adolescents and young adults undergoing HSCT for acute myelogenous leukemia, no difference in risk was seen among survivors for 10-year cumulative incidence of myocardial infarction or congestive heart failure regardless of whether they received total body irradiation during the conditioning phase.24
Post-HSCT subclinical cardiac injury has been reported in pediatric patients. In 95 children surviving at least 1 year post-HSCT, cardiac biomarkers were elevated in the first 49 days after transplantation (soluble suppression of tumorigenicity-2 and troponin I) despite normal echocardiography.25 Whether this equates a long-term risk to develop clinically evident dysfunction is not known but suggests the need for some level of prescribed long-term follow-up.
Risk Models
Risk prediction models for cardiac late effects after treatment for cancer and for HSCT have been proposed.26 Much of these data come from the Childhood Cancer Survivor Study, which follows patients treated as children in the 1970s and 90s for a heterogeneous group of cancers, including a small proportion that underwent HSCT. Armenian et al. have created a tool to estimate the risk of heart failure and coronary artery disease by 10 years post HSCT, with variables that significantly impact this risk including age, anthracycline dose, chest radiation, hypertension, diabetes, and smoking history.14 Risk scores are generated to represent low-, intermediate-, and high-risk groups corresponding to 10-year cumulative incidences of cardiovascular disease of 3.7%, 9.9%, and 26.2%, respectively.14 Compared to individuals in the low-risk group, those considered intermediate- and high-risk were 2.9-fold and 7.8-fold more likely to develop cardiovascular disease on follow-up.14
The Charlson Comorbidity Index (CCI) was proposed as an easily derivable score to account for pre-existing co-morbidities in predicting outcomes in patients undergoing allogeneic HSCT, but not all risk was captured.27,28 An alternative score based on the CCI, the Hematopoietic Cell Transplantation Comorbidity Index, assigned a weighted score based on the presence of several cardiovascular parameters: coronary artery disease, congestive heart failure, history of myocardial infarction or ejection fraction <50%, and other comorbidities.29 Although the Hematopoietic Cell Transplantation Comorbidity Index performed better than CCI in predicting non-relapse mortality at 2 years (C-statistic of 0.685 compared to 0.532 for CCI), its discriminatory performance was still only modest. Neither of these scoring systems was developed specifically in pediatric patients.
Conclusion
Cardiac complications may occur in both pediatric and adult patients after HSCT, both in the short and long term. Pre-HSCT cardiac comorbidities are common, particularly in adults. Even in patients undergoing HSCT with no known cardiac disease, use of radiation and anthracycline chemotherapy as part of pre-HSCT malignancy treatment may predispose to events occurring during and after HSCT. Long-term surveillance should be considered, particularly in those patients with known risk factors.
References
- D'Souza A, Lee S, Zhu X, Pasquini M. Current Use and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant 2017;23:1417-21.
- Khandelwal P, Millard HR, Thiel E, et al. Hematopoietic Stem Cell Transplantation Activity in Pediatric Cancer between 2008 and 2014 in the United States: A Center for International Blood and Marrow Transplant Research Report. Biol Blood Marrow Transplant 2017;23:1342-9.
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med 2006;354:1813-26.
- Rotz SJ, O'Riordan MA, Kim C, de Lima M, Gladwin MT, Little JA. Traffic Light: prognosis-based eligibility for clinical trials of hematopoietic SCT in adults with sickle cell anemia. Bone Marrow Transplant 2015;50:918-23.
- D'Souza A, Fretham C. Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT) (Center for International Blood & Marrow Transplant Research website). 2017. Available at http://www.cibmtr.org. Accessed December 17, 2020.
- Rotz SJ, Ryan TD, Hayek SS. Cardiovascular disease and its management in children and adults undergoing hematopoietic stem cell transplantation. J Thromb Thrombolysis 2020;Nov 24:[Epub ahead of print].
- Buja LM, Ferrans VJ, Graw RG Jr. Cardiac pathologic findings in patients treated with bone marrow transplantation. Hum Pathol 1976;7:17-45.
- Armenian SH, Sun CL, Shannon T, et al. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood 2011;118:6023-9.
- Armenian SH, Sun CL, Francisco L, et al. Late congestive heart failure after hematopoietic cell transplantation. J Clin Oncol 2008;26:5537-43.
- Leger KJ, Baker KS, Cushing-Haugen KL, et al. Lifestyle factors and subsequent ischemic heart disease risk after hematopoietic cell transplantation. Cancer 2018;124:1507-15.
- Leger KJ, Cushing-Haugen K, Hansen JA, et al. Clinical and Genetic Determinants of Cardiomyopathy Risk among Hematopoietic Cell Transplantation Survivors. Biol Blood Marrow Transplant 2016;22:1094-101.
- Tichelli A, Bucher C, Rovó A, et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood 2007;110:3463-71.
- Chow EJ, Mueller BA, Baker KS, et al. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med 2011;155:21-32.
- Armenian SH, Yang D, Teh JB, et al. Prediction of cardiovascular disease among hematopoietic cell transplantation survivors. Blood Adv 2018;2:1756-64.
- DeFilipp Z, Duarte RF, Snowden JA, et al. Metabolic syndrome and cardiovascular disease following hematopoietic cell transplantation: screening and preventive practice recommendations from CIBMTR and EBMT. Bone Marrow Transplant 2017;52:173-82.
- Gibson CJ, Steensma DP. New Insights from Studies of Clonal Hematopoiesis. Clin Cancer Res 2018;24:4633-42.
- Gibson CJ, Lindsley RC, Tchekmedyian V, et al. Clonal Hematopoiesis Associated With Adverse Outcomes After Autologous Stem-Cell Transplantation for Lymphoma. J Clin Oncol 2017;35:1598-605.
- Jaiswal S, Natarajan P, Silver AJ, et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med 2017;377:111-21.
- Frick M, Chan W, Arends CM, et al. Role of Donor Clonal Hematopoiesis in Allogeneic Hematopoietic Stem-Cell Transplantation. J Clin Oncol 2019;37:375-85.
- Collord G, Park N, Podestà M, et al. Clonal haematopoiesis is not prevalent in survivors of childhood cancer. Br J Haematol 2018;181:537-9.
- Duncan CN, Brazauskas R, Huang J, et al. Late cardiovascular morbidity and mortality following pediatric allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2018;53:1278-87.
- Uderzo C, Pillon M, Corti P, et al. Impact of cumulative anthracycline dose, preparative regimen and chronic graft-versus-host disease on pulmonary and cardiac function in children 5 years after allogeneic hematopoietic stem cell transplantation: a prospective evaluation on behalf of the EBMT Pediatric Diseases and Late Effects Working Parties. Bone Marrow Transplant 2007;39:667-75.
- Rotz SJ, Powell A, Myers KC, et al. Treatment exposures stratify need for echocardiographic screening in asymptomatic long-term survivors of hematopoietic stem cell transplantation. Cardiol Young 2019;29:338-43.
- Lee CJ, Kim S, Tecca HR, et al. Late effects after ablative allogeneic stem cell transplantation for adolescent and young adult acute myeloid leukemia. Blood Adv 2020;4:983-92.
- Rotz SJ, Dandoy CE, Taylor MD, et al. Long-term systolic function in children and young adults after hematopoietic stem cell transplant. Bone Marrow Transplant 2017;52:1443-7.
- Leerink JM, de Baat EC, Feijen EA, et al. Cardiac Disease in Childhood Cancer Survivors: Risk Prediction, Prevention, and Surveillance: JACC CardioOncology State-of-the-Art Review. JACC CardioOncol 2020;2:363-78.
- Sorror ML, Maris MB, Storer B, et al. Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative and myeloablative conditioning: influence of pretransplantation comorbidities. Blood 2004;104:961-8.
- Diaconescu R, Flowers CR, Storer B, et al. Morbidity and mortality with nonmyeloablative compared with myeloablative conditioning before hematopoietic cell transplantation from HLA-matched related donors. Blood 2004;104:1550-8.
- Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005;106:2912-9.
Clinical Topics: Anticoagulation Management, Arrhythmias and Clinical EP, Cardio-Oncology, Diabetes and Cardiometabolic Disease, Dyslipidemia, Heart Failure and Cardiomyopathies, Noninvasive Imaging, Pericardial Disease, Prevention, Pulmonary Hypertension and Venous Thromboembolism, Vascular Medicine, Atherosclerotic Disease (CAD/PAD), Implantable Devices, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Acute Heart Failure, Heart Failure and Cardiac Biomarkers, Pulmonary Hypertension, Echocardiography/Ultrasound, Hypertension
Keywords: Cardiotoxicity, Cardio-oncology, Troponin I, Graft vs Host Disease, Cardiovascular Diseases, Whole-Body Irradiation, Anthracyclines, Anemia, Aplastic, Multiple Myeloma, Mucositis, Coronary Artery Disease, Case-Control Studies, Bone Marrow, Hepatic Veno-Occlusive Disease, Hypertension, Pulmonary, Pericardial Effusion, Factor XIII, Factor X, Factor XI, Fibrin, Transplantation, Autologous, Confidence Intervals, Follow-Up Studies, Predictive Value of Tests, Stroke Volume, Heart Failure, Hematopoietic Stem Cell Transplantation, Lymphoma, Leukemia, Myeloid, Acute, Myocardial Infarction, Diabetes Mellitus, Cardiomyopathies, Lymphocytes, Risk Factors, Cardiovascular System, Arrhythmias, Cardiac, Blood Cells, Erythrocytes, Dyslipidemias, Hematopoietic Stem Cells, Cyclophosphamide, Metabolic Diseases, Thrombotic Microangiopathies, Inflammation, Lung Diseases, Interstitial, Hemoglobinopathies, Transplantation, Homologous, Macrophages, Malnutrition, Disease Eradication, Bone Marrow Cells, Acute Kidney Injury, Echocardiography, Biomarkers, Necrosis, Obesity
< Back to Listings