Cryoablation or Drug Therapy for Initial Treatment of AF (The EARLY-AF Trial)
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia encountered in clinical practice. In addition to reductions in quality of life, functional status, and cardiac performance, AF is associated with an increased risk of stroke and reduced survival. The contemporary management of AF is centered on improving arrhythmia-related symptoms and reducing the morbidity and mortality associated with AF. Over the past 15 years, catheter ablation has emerged as an important non-pharmacological treatment option for highly symptomatic patients in whom antiarrhythmic drugs (AADs) are ineffective or poorly tolerated.1-4 However, these studies were performed in patients who had already failed AAD therapy, weighting the benefit toward ablation. Relatively few studies have compared ablation to AADs as the initial therapy.5-7
Previous studies have attempted to answer whether a population may exist for whom the effectiveness of the procedure would be sufficiently high and the risks sufficiently low that it would be appropriate to offer AF ablation as first-line therapy. Prior studies of first-line radiofrequency catheter ablation have been limited by the use of intermittent noninvasive rhythm monitoring and high rates of cross-over from AADs to ablation (25-28%), blunting the ability to detect a difference between treatment groups.5-7 In aggregate, the 3 randomized studies of first-line radiofrequency ablation reported relatively low absolute success rates (45.5-52.7% freedom from atrial tachyarrhythmia in the ablation arm vs. 27.9-43.9% in the AAD arm) with a low aggregate relative benefit with first-line radiofrequency ablation (relative risk [RR] 0.81 for any arrhythmia; 95% confidence interval [CI], 0.68-0.96; p = 0.01; and RR 0.62 for symptomatic arrhythmia; 95% CI, 0.38-1.01; p = 0.06), which, when combined with the lack of procedural standardization and inconsistent procedural endpoints, have limited the impact of these studies.5-7
Guideline Recommendations
Given the limitations of the previous randomized studies, the major North American and European society guidelines provide only a conditional recommendation for AF ablation as first-line therapy, reserving it for highly selected patients with symptomatic paroxysmal (Class IIa in European Society of Cardiology [ESC] and American Heart Association [AHA]/American College of Cardiology [ACC]/Heart Rhythm Society [HRS] guidelines) or persistent AF (Class IIb in ESC and AHA/ACC/HRS guidelines) without major risk factors for recurrence, considering patient preference, benefit, and risk.8,9 The Canadian Cardiovascular Society guidelines recommend first-line catheter ablation in select patients with symptomatic AF, with no distinction between paroxysmal or persistent AF (Weak Recommendation).10
Study Rationale and Design
The EARLY-AF (Early Aggressive Invasive Intervention for Atrial Fibrillation) trial sought to evaluate the role of cryoballoon ablation versus AADs in the first-line treatment of AF in AAD-naïve patients.11 Patients randomized to cryoballoon ablation underwent circumferential pulmonary vein isolation using a second-generation cryoballoon, with a procedure endpoint of bidirectional block after a 20-minute observation period. Patients randomized to AADs had their AADs aggressively optimized using standardized titration protocols.12 In contrast to previous studies, all patients in the EARLY-AF trial received an implantable cardiac monitor for continuous rhythm monitoring, with all arrhythmia events undergoing independent adjudication by a committee blinded to treatment allocation.
Primary and Secondary Outcomes
The primary outcome was the first recurrence of any atrial tachyarrhythmia, defined as AF, atrial flutter, or atrial tachycardia lasting 30 seconds or longer, between 91 and 365 days after treatment (catheter ablation or AAD initiation) initiation. Secondary outcomes included the first recurrence of symptomatic atrial tachyarrhythmia between 91 and 365 days, total arrhythmia burden, disease-specific and generic quality of life, health care utilization, and serious adverse events. Serious adverse events included events causing death or functional disability, warranting intervention, or resulting in or prolonging hospitalization for more than 24 hours.
Pertinent Findings
The EARLY-AF study enrolled 303 patients, randomizing 154 to undergo cryoballoon ablation and 149 to receive AAD therapy.12 Among the 152 patients who underwent ablation, complete pulmonary vein isolation was confirmed in all patients, with a median procedure duration of 106 minutes (interquartile range [IQR] 89-131). Among patients randomized to AAD therapy, the most frequently prescribed AAD was flecainide at a median daily dose of 200 mg per day (IQR 125-250).
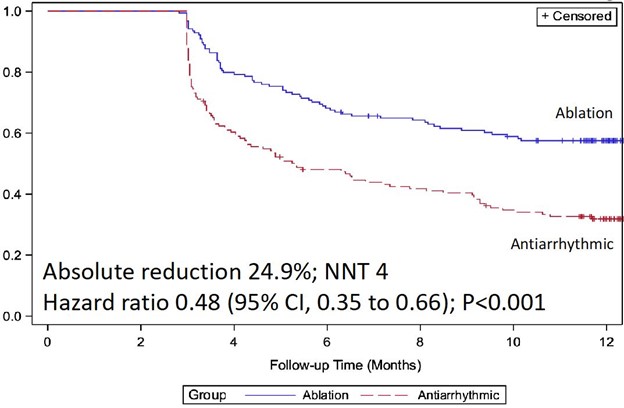
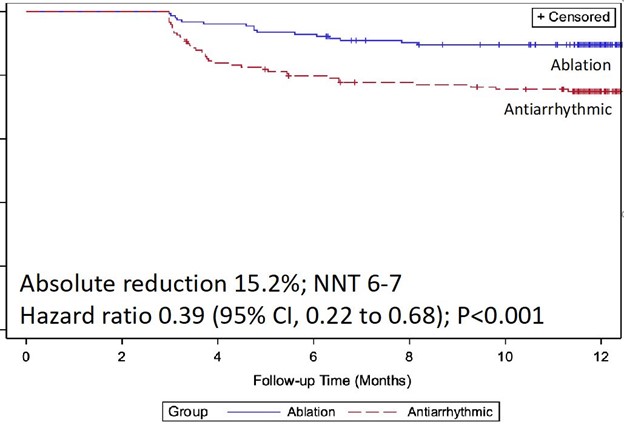
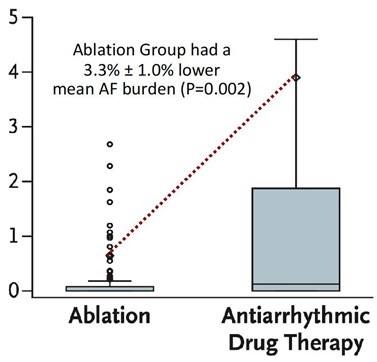
At 1 year following treatment initiation, documented recurrence of atrial tachyarrhythmia occurred in 42.9% of patients randomized to cryoballoon ablation and 67.8% of patients randomized to AADs (hazard ratio 0.48; 95% CI, 0.35-0.66; p < 0.001) (Figure 1).12 Symptomatic atrial tachyarrhythmia at 1 year occurred in 11.0% of patients randomized to ablation and 26.2% of patients randomized to AADs (hazard ratio 0.39; 95% CI, 0.22-0.68) (Figure 2). The median AF burden was 0% (IQR 0-0.08) in patients randomized to ablation and 0.13% (IQR 0-1.60) in patients randomized to AADs (Figure 3).
Figure 1: Freedom From Any Atrial Tachyarrhythmia
Figure 2: Freedom From Symptomatic Atrial Tachyarrhythmia
Figure 3: AF Burden in Patients Treated With First-Line Ablation vs. First-Line AAD Therapy
At 1-year follow-up, the least-squares mean change from baseline score on the AF Effect on Quality-of-Life (AFEQT) survey was +26.9 ± 1.9 points in patients randomized to ablation and +22.9 ± 2.0 points in patients randomized to AADs, with a mean treatment effect (i.e., difference between randomized groups) of 8.0 ± 2.2 points (Table 1). The proportion of asymptomatic patients at 1 year was 85.1% of patients randomized to ablation and 73.2% of patients randomized to AADs (RR 1.17; 95% CI, 1.05-1.30).
Serious adverse events occurred in 3.2% of patients randomized to ablation and 4.0% of patients randomized to AADs, with no significant difference between groups.
Table 1: Primary and Secondary Outcomes
| Endpoint | Ablation Group (n = 154) |
Antiarrhythmic Group (n = 149) |
Treatment Effect (95% CI) |
| Primary endpoint Recurrence of any atrial tachyarrhythmia |
66 (42.9) |
101 (67.8) |
0.48 (0.35, 0.66) |
| Secondary endpoints Recurrence of symptomatic atrial tachyarrhythmia AF burden, median (IQR) AF burden, mean |
17 (11.0) 0 (0, 0.08) 0.6 ± 3.3 |
39 (26.2) 0.13 (0, 1.60) 3.9 ± 12.4 |
0.39 (0.22, 0.68) -3.3 ± 1.0 |
| Quality-of-life endpoints Change from baseline AFEQT score at 6 months Change from baseline AFEQT score at 12 months No symptoms at 6 months No symptoms at 12 months |
24.4 ± 1.6 26.9 ± 1.9 129 (83.8) 131 (85.1) |
17.9 ± 1.6 22.9 ± 2.0 90 (60.4) 109 (73.2) |
10.5 ± 2.2 8.0 ± 2.2 1.34 (1.17, 1.55) 1.17 (1.05, 1.30) |
| Safety endpoints Any serious adverse event related to trial regimen |
5 (3.2) |
6 (4.0) |
0.81 (0.25, 2.59) |
Discussion
The recent EAST-AFNET 4 (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial) has highlighted the role of early rhythm control for patients with newly diagnosed AF. Specifically, early pharmacological rhythm control significantly reduced the composite primary outcome of cardiovascular death, stroke, and hospitalization for worsening heart failure and acute coronary syndrome, providing a reasonable justification to pursue early rhythm control in patients with a recent diagnosis of AF.13 Effectively, EAST-AFNET 4 has answered the question of why we should pursue rhythm control for patients with newly diagnosed AF.
Complementarily, the EARLY-AF trial focuses on the question of how rhythm control can be effectively achieved in highly symptomatic patients with treatment-naïve AF. The EARLY-AF trial has demonstrated that first-line cryoballoon ablation was superior to AADs for the outcomes of atrial tachyarrhythmia recurrence, arrhythmia burden, and quality of life. In terms of arrhythmia recurrence, the number needed to treat was 4 to prevent any atrial tachyarrhythmia recurrence and 6 to prevent symptomatic atrial tachyarrhythmia recurrence. In terms of AF burden, the mean difference between groups corresponded to the equivalent of 1 fewer day with AF per month. In terms of quality of life, the difference between treatments groups more than twice exceeded the minimally clinically relevant difference (i.e., 5 points on AFEQT score). Although the overall outcome of health care utilization was not significantly different between randomized groups, there was a numerical reduction in health care utilization events (cardioversion, emergency department visits, hospitalization) in the first-line ablation group, with a trend toward reduction for hospitalization (p = 0.05).
The design of the EARLY-AF trial included several unique features to address the aforementioned limitations of the previous first-line ablation studies. Specifically, although intermittent noninvasive rhythm monitoring is the most widely used method of ascertaining arrhythmia recurrence, it lacks sensitivity in detecting sporadic arrhythmias (e.g., paroxysmal AF), leading to under-detection of recurrences and introducing misclassification errors that could potentially impact the accuracy and precision of comparative risk estimates. Instead, the EARLY-AF trial relied on implantable loop recorders to precisely determine the presence and timing of arrhythmia recurrence, as well as examine quantitative outcomes such as arrhythmia burden. Second, in an effort to ensure that the treatment comparison was robust, the dose of AADs in the pharmacotherapy group was aggressively up-titrated using standardized protocols over a 3-month treatment optimization period with a goal of complete AF suppression on loop recorder monitoring. Third, the trial employed pre-specified protocols, including the establishment of an independent committee, in an effort to ensure that no patient in the EARLY-AF trial crossed over between randomized groups prior to experiencing a primary endpoint event.
It is postulated that an early intervention may have beneficial effects on progression to more-persistent forms of AF, but this question could not be addressed in the current study because follow-up was limited to 12 months. Additional studies will be needed to determine the longer-term impact of early catheter ablation on the progression of AF, health care utilization, cost-effectiveness, and patient-reported outcomes. In addition, the EARLY-AF trial was performed with a single ablation technology. Although previous studies have suggested similar outcomes with contact-force radiofrequency ablation and cryoballoon ablation,14 the results of the EARLY-AF trial may not be generalizable to the use of other ablation energy sources or technologies. Lastly, the quality-of-life results demonstrated superiority of the ablation strategy over AADs, although blinding of participants to the treatment strategy cannot be achieved without a sham procedure.
Conclusions
A strategy of first-line cryoballoon ablation was superior to AADs for the treatment of patients with drug-naïve AF. Compared to AADs, cryoballoon ablation is demonstrably superior in freedom from atrial tachyarrhythmia recurrence, superior in reduction in AF burden, and superior in improvement in quality of life. These findings are relevant to inform patients, providers, and health care systems regarding the choice of rhythm-control therapy in patients with drug treatment-naïve AF.
References
- Pappone C, Augello G, Sala S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study. J Am Coll Cardiol 2006;48:2340-7.
- Jaïs P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008;118:2498-505.
- Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333-40.
- Stabile G, Bertaglia E, Senatore G, et al. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation For The Cure Of Atrial Fibrillation Study). Eur Heart J 2006;27:216-21.
- Cosedis Nielsen J, Johannessen A, Raatikainen P, et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med 2012;367:1587-95.
- Morillo CA, Verma A, Connolly SJ, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA 2014;311:692-700.
- Wazni OM, Marrouche NF, Martin DO, et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA 2005;293:2634-40.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:e199-267.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation 2019;140:e125-e151.
- Andrade JG, Aguilar M, Atzema C, et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Comprehensive Guidelines for the Management of Atrial Fibrillation. Can J Cardiol 2020;36:1847-948.
- Andrade JG, Champagne J, Deyell MW, et al. A randomized clinical trial of early invasive intervention for atrial fibrillation (EARLY-AF) - methods and rationale. Am Heart J 2018;206:94-104.
- Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or Drug Therapy for Initial Treatment of Atrial Fibrillation. N Engl J Med 2020;384:305-15.
- Kirchhof P, Camm AJ, Goette A, et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N Engl J Med 2020;383:1305-16.
- Andrade JG, Champagne J, Dubuc M, et al. Cryoballoon or Radiofrequency Ablation for Atrial Fibrillation Assessed by Continuous Monitoring: A Randomized Clinical Trial. Circulation 2019;140:1779-88.
Clinical Topics: Arrhythmias and Clinical EP, Cardiovascular Care Team, Implantable Devices, EP Basic Science, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias
Keywords: Arrhythmias, Cardiac, Anti-Arrhythmia Agents, Flecainide, Quality of Life, Pulmonary Veins, Atrial Fibrillation, Atrial Flutter, American Heart Association, Confidence Intervals, Patient Preference, Catheter Ablation, Tachycardia, Supraventricular, Electrocardiography, Stroke, Risk Factors, Morbidity, Patient Acceptance of Health Care, Reference Standards, Hospitalization, Family Characteristics
< Back to Listings