An Angiographic Risk Score for 12-Month Restenosis in Femoropopliteal Intervention
Quick Takes
- The current study evaluated the 12-month restenosis risk from the viewpoint of anatomical severity and identified cases that were expected to be at high risk for 12-month restenosis after femoropopliteal (FP)-endovascular therapy (EVT).
- The risk analysis identified small vessel diameter, long lesions, and chronic total occlusion (CTO) as independent angiographic risk factors.
- A simple angiographic score, developed from these risk factors, was significantly and independently associated with 12-month restenosis risk, whereas the Trans-Atlantic Inter-Society Consensus (TASC) II classification did not.
Paclitaxel (PTX)-coated devices are reliable tools to reduce restenosis risk and improve long-term vessel patency after femoropopliteal (FP)-endovascular therapy (EVT) for patients with peripheral artery disease (PAD).1-3 However, a recent meta-analysis suggested that these devices could possibly increase the risk of long-term mortality.4 Regulators now recommend limiting the use to patients who are expected to be at high risk for restenosis after conventional EVT. However, the classification of patients who are "at high risk" is not well defined. Therefore, this study aimed to identify anatomical features associated with the 12-month restenosis risk after FP-EVT in the current real-world clinical practice, and to develop a novel angiographic scoring system for risk stratification.
The current study used a clinical database called the IntraVascular UltrasOund-SuppoRted Endovascular Therapy in Superficial Femoral ArterY Disease (IVORY) registry. IVORY is a prospective multicenter observational study that registered adult patients in whom intravascular ultrasound-supported FP-EVT was planned for symptomatic atherosclerotic PAD (Rutherford category 1 to 6) between November 2015 and June 2017. Registration was in advance of EVT, and patients in whom bypass surgery and/or major amputation after EVT was originally planned were excluded. The study subjects were enrolled in 33 participating centers across Japan and were followed for 1 year. In total, 2018 limbs from 1,766 patients were enrolled. After enrollment, two patients were excluded due to residual inflow stenosis, another was excluded due to a lack of angiographically significant stenosis, and a further patient had unsuccessful wire crossing. Also excluded were cases treated with drug-coated balloons (n=8) due to the small sample size, those treated with different kinds of stents (n=28), and those not taking two or more antithrombotic drugs (n=179). Consequently, the current study analyzed the remaining 1,799 limbs from 1,578 patients. The study endpoint was 12-month primary patency (i.e., freedom from restenosis) as assessed by duplex ultrasound or follow-up angiography, with a range of ±2 months. Restenosis was defined as a peak systolic velocity ratio >2.4 according to duplex ultrasound or the recurrence of stenosis ≥50% of the arterial diameter, as determined by angiography. The need for any reintervention or major amputation (defined as surgical limb removal above the ankle) within 1 year was automatically counted as restenosis. The risk factors for 12-month restenosis were explored with a generalized linear mixed model with a logit-link function in which interinstitutional and intersubject variability were treated as the random effects. The multiple imputation method was adopted to address missing data.
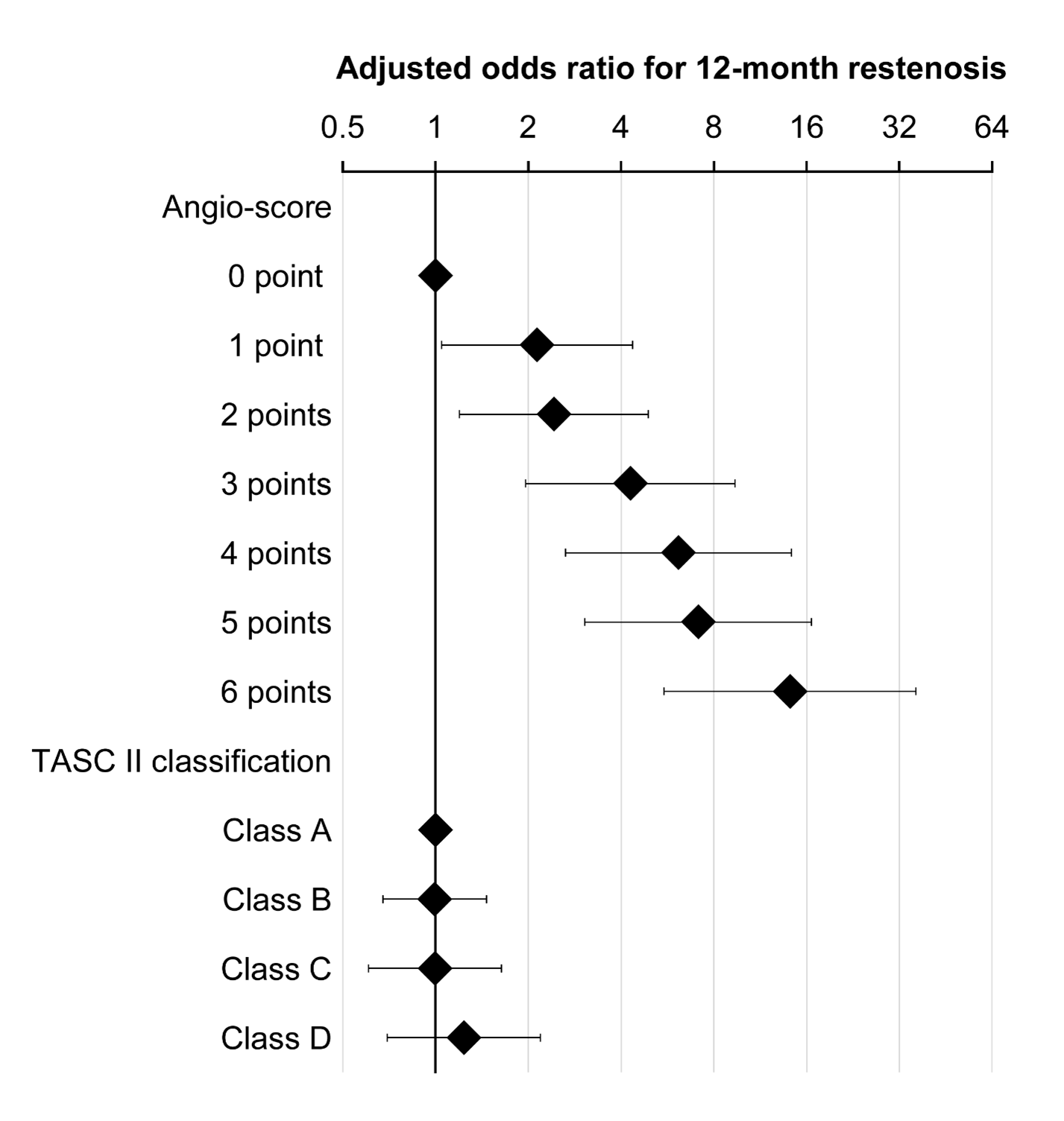
The patients were 74 ± 9 years old, 69.1% were male, and chronic limb-threatening ischemia was found in 24.6%. The mean distal reference vessel diameter was 5.0 ± 1.0 mm, the mean lesion length was 16 ± 10 cm, and the prevalence of chronic total occlusion (CTO) was 39.7%. The primary patency at 12 ± 2 months was assessed in 80.3% of the limbs (n=1,444). Patency was confirmed in 941 cases, whereas 503 cases were diagnosed as restenosis. The proportion of 12-month primary patency was estimated to be 65.1% (95% CI: 62.7%-67.5%). After multivariate analysis, baseline patient characteristics were not independently associated with 12-month restenosis risk, whereas the following three angiographical findings were independently associated with 12-month restenosis risk: distal reference vessel diameter, lesion length, and CTO (all P<0.01). The adjusted odds ratios (95% confidence interval) were 1.41 [1.23-1.60] per 1-mm decrease, 1.39 (1.19 to 1.62) per 10-cm increase, and 1.56 (1.15 to 2.10), respectively. Compared to bare metal stent implantation, plain angioplasty was independently associated with a higher risk of 12-month restenosis (P<0.001), whereas drug-eluting stent implantation and stent graft implantation were independently associated with a lower risk of 12-month restenosis (both P<0.05). Using the three angiographic risk factors, we developed a lesion-related risk score (called "angio-score") as follows: [long lesion (0, 1, 2, and 3 points were given for ≤5, 5-15, 15-25, and >25 cm)] + small distal reference vessel diameter (0, 1, and 2 points were given for ≥5.5, 4.5-5.5, and <4.5 cm)] + [CTO (0 and 1 point were given for nonocclusive and occlusive lesions, respectively)]. This angio-score was associated with a graded increased risk of 12-month restenosis, while TASC II classification was not (Figure 1).
Figure 1
We concluded that a novel angiographic score, which includes reference vessel diameter, lesion length, and CTO, is a useful tool to assess 12-month restenosis risk after femoropopliteal EVT in a real-world clinical practice.
References
- Dake MD, Ansel GM, Jaff MR, et al. Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the Zilver PTX randomized trial. Circulation 2016;133:1472-83.
- Schneider PA, Laird JR, Tepe G, et al. Treatment effect of drug-coated balloons is durable to 3 years in the femoropopliteal arteries: long-term results of the IN.PACT SFA randomized trial. Circ Cardiovasc Interv 2018;11:e005891.
- Gray WA, Keirse K, Soga Y, et al. A polymer-coated, paclitaxel-eluting stent (Eluvia) versus a polymer-free, paclitaxel-coated stent (Zilver PTX) for endovascular femoropopliteal intervention (IMPERIAL): a randomised, non-inferiority trial. Lancet 2018;392:1541-51.
- Katsanos AH, Malhotra K, Goyal N, et al. Mortality risk in acute ischemic stroke patients with large vessel occlusion treated with mechanical thrombectomy. J Am Heart Assoc 2019;8:e014425.
Clinical Topics: Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Vascular Medicine, Atherosclerotic Disease (CAD/PAD), Interventions and Imaging, Interventions and Vascular Medicine, Angiography, Echocardiography/Ultrasound, Nuclear Imaging
Keywords: Drug-Eluting Stents, Fibrinolytic Agents, Femoral Artery, Peripheral Arterial Disease, Constriction, Pathologic, Odds Ratio, Paclitaxel, Confidence Intervals, Sample Size, Prospective Studies, Follow-Up Studies, Angioplasty, Stents, Ischemia, Angiography, Risk Factors, Multivariate Analysis, Ultrasonography, Interventional, Registries, Risk Assessment, Pharmaceutical Preparations
< Back to Listings