Downstream or Upstream P2Y12 Receptor Blockers in NSTE-ACS: Primary Results of the DUBIUS Trial
Quick Takes
- The available evidence related to the clinical impact of pretreatment has been incomplete and heterogeneous regarding the different P2Y12 inhibitors.
- In the DUBIUS (Downstream Versus Upstream Strategy for the Administration of P2Y12 Receptor Blockers in Non-ST Elevated Acute Coronary Syndromes With Initial Invasive Indication) trial, downstream and upstream oral P2Y12 inhibitor administration strategies were associated with low incidence of ischemic and bleeding events and minimal numeric difference of event rates between treatment groups.
- Broad use of a radial approach may have contributed to the low observed adverse event rates.
Introduction
Dual antiplatelet therapy with aspirin and an oral P2Y12 inhibitor is the standard treatment in patients with non-ST-segment elevation acute coronary syndrome (NSTE-ACS). Among P2Y12 inhibitors, ticagrelor and prasugrel are associated with better clinical outcomes compared with clopidogrel in patients with acute coronary syndrome (ACS) and are thus preferred unless contraindicated.1 In recent years, the timing of P2Y12 inhibitor administration has been a matter of debate.2 Their administration before coronary angiography (also known as pretreatment) could have the potential to reduce periprocedural ischemic events in patients undergoing percutaneous coronary intervention (PCI), but this strategy may also increase the risk of major bleeding. Moreover, the available evidence related to the clinical impact of pretreatment has been incomplete and heterogeneous regarding the different P2Y12 inhibitors.2
Prior Studies
The concept of early initiation of dual antiplatelet therapy in patients with NSTE-ACS originally derived from trials on clopidogrel: CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial) and the CREDO (Clopidogrel for the Reduction of Events During Observation) trial. However, a large proportion of patients in CURE did not undergo coronary angiography, and among those treated invasively, the median interval between enrollment and angiography was several days. In the CREDO trial, patients were selected for enrollment after assessing coronary anatomy by angiography.
The PLATO (Platelet Inhibition and Patient Outcomes) study randomized 18,624 patients with ACS to receive ticagrelor or clopidogrel. Based on the observed early benefit of ticagrelor over clopidogrel in terms of ischemic events (without an increase in the rate of overall major bleeding), the PLATO study provided evidence in favor of early initiation of ticagrelor therapy. However, the study drugs were administered before coronary angiography, and the study did not specifically investigate the issue of pretreatment.
TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel–Thrombolysis In Myocardial Infarction 38) randomized 13,608 patients with ACS to receive prasugrel or clopidogrel. Prasugrel reduced the rates of ischemic events but with an increased risk of major bleeding. However, patients in this trial received prasugrel after coronary angiography if the indication for PCI was confirmed.
The ACCOAST study (A Comparison of Prasugrel at PCI or Time of Diagnosis of Non-ST Elevation Myocardial Infarction) specifically evaluated pretreatment with prasugrel. A total of 4,033 patients was randomized to receive prasugrel (pretreatment group) or a placebo (control group) before coronary angiography. Pretreatment with prasugrel was associated with an increased rate of major bleeding without reducing the rate of major ischemic events up to 30 days.
The ISAR-REACT 5 study (Intracoronary Stenting and Antithrombotic Regimen 5) randomized 4,018 patients with ACS to receive ticagrelor or prasugrel.3 In the ticagrelor group, the loading dose was administered as soon as possible after randomization. In the prasugrel group, the loading dose was administered as soon as possible in patients with ST-segment elevation ACS (41%) and after coronary anatomy was known in the patients with NSTE-ACS. Prasugrel significantly reduced ischemic events without an increase in the rate of major bleeding at 1 year. Due to such study design, both the study drugs and the administration strategy may have contributed to the observed outcomes with a mixed effect.
The DUBIUS Trial Study Design
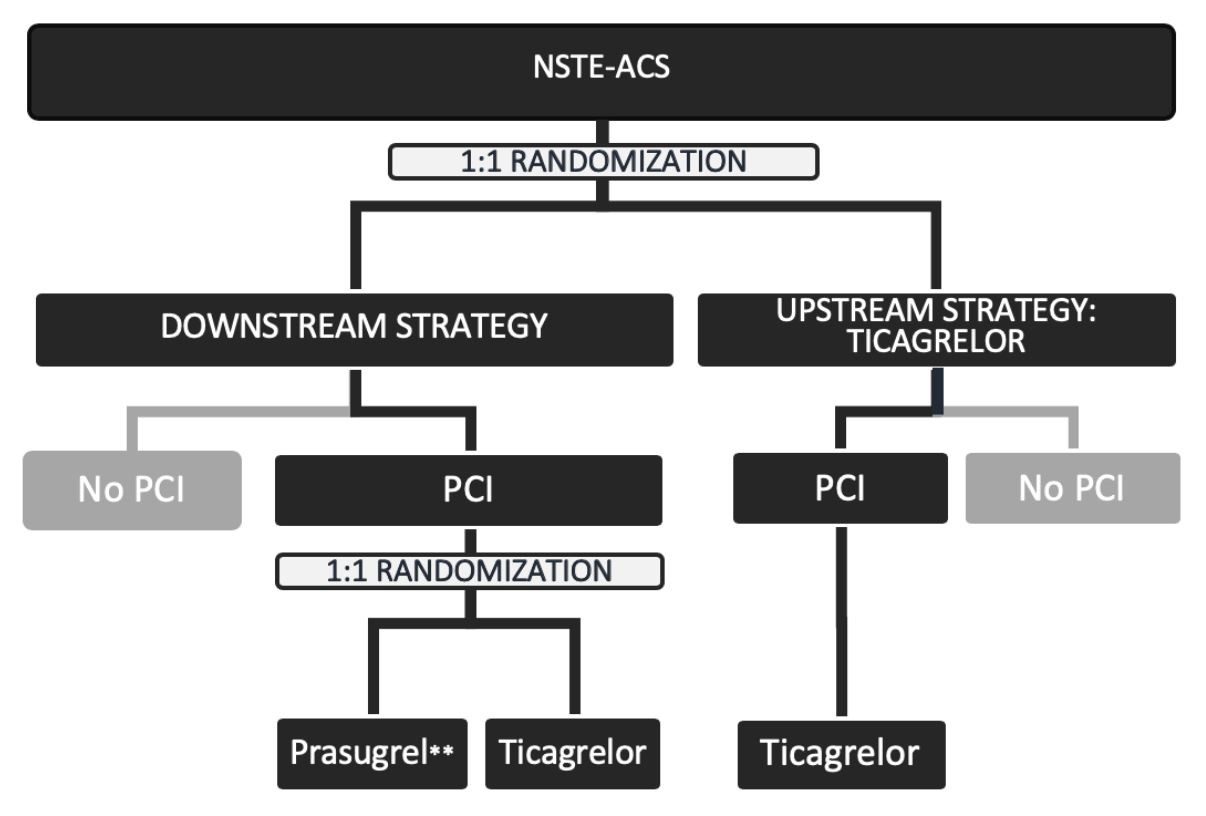
The DUBIUS trial is a randomized, adaptive, open-label, and multicenter clinical trial that sought to compare downstream and upstream oral P2Y12 inhibitor administration strategies in patients with NSTE-ACS undergoing invasive treatment.4,5 Patients were randomly assigned to pretreatment with ticagrelor before angiography (upstream group) or no pretreatment (downstream group). In the downstream group, all patients undergoing PCI were further randomized to the administration of ticagrelor or prasugrel.
The primary hypothesis was that there would be superiority of the downstream versus the upstream administration strategy in terms of the net clinical benefit on ischemic and haemorrhagic events. The primary endpoint was a composite of death from vascular causes (death from cardiovascular causes or cerebrovascular causes and any death without another known cause), nonfatal myocardial infarction, or nonfatal stroke and major or fatal bleeding (types 3, 4, and 5 on the BARC scale) at 30 days after randomization.
Primary Results of the DUBIUS Trial
The study randomized 1,449 patients from December 2015 through May 2020 from 30 centers in Italy. At the second interim analysis, the incidence of the primary endpoint was assessed. Based on a prespecified stopping rule, the steering committee decided to stop enrollment for a futility scenario. Patient characteristics were well-balanced across study arms. The median GRACE score was 122 in both groups, and the median CRUSADE score was 22 in the downstream group and 21 in the upstream group. More than 99% of patients underwent coronary angiography (median time of 23.3 hours), mostly by the radial approach (94.5% of patients). Revascularization was performed by PCI in 72% of patients and by coronary artery bypass graft in 6%. No revascularization was required in 22% of patients.
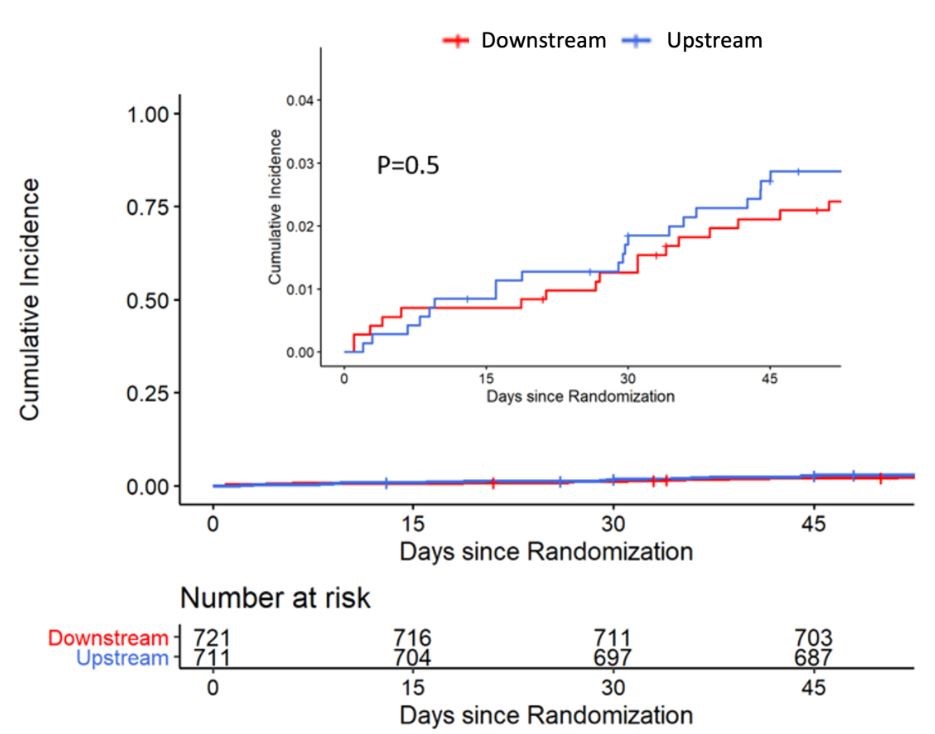
No differences in the rate of the primary endpoint were observed between the downstream and upstream groups (2.9% and 3.3%, respectively; absolute risk reduction –0.46; 95% confidence interval, –2.87 to 1.89). The most common adverse events were major or fatal bleedings, with no differences between groups. The results were independent from the timing of coronary angiography (within or after 24 hours from enrollment) and from treatment with or without PCI. As an exploratory analysis, within the PCI subgroup of the downstream arm, the rate of the primary endpoint did not significantly differ between patients treated with prasugrel and those treated with ticagrelor (4.1% and 3.1%, respectively; absolute risk reduction 0.9; 95% confidence interval, –3 to 5).
Clinical Implications
The DUBIUS trial is the first study specifically designed to test pretreatment with ticagrelor in patients with NSTE-ACS. The DUBIUS trial was prematurely stopped after an interim analysis due to a futility scenario, given the unlikelihood to detect a benefit of one strategy over the other with a continuation of enrollments. Even if the findings should be considered exploratory, the DUBIUS trial showed that in patients with NSTE-ACS with planned invasive treatment, both a downstream treatment strategy with an oral P2Y12 inhibitor (prasugrel or ticagrelor) and a ticagrelor-based pretreatment strategy are associated with low rates of ischemic and bleeding events. Several factors may have contributed to the low adverse event rates observed in the trial, including early timing of coronary angiography and revascularization, very high rates of radial approach, and broad implementation of secondary prevention measures.
Summary
In patients with NSTE-ACS undergoing early invasive treatment, downstream and upstream oral P2Y12 inhibitor administration strategies were associated with low incidence of ischemic and bleeding events and minimal numeric difference of event rates between treatment groups. Broad use of a radial approach may have contributed to the low observed adverse event rates.
Figure 1
Figure 2
References
- Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2020;Aug 29:[Epub ahead of print].
- Capodanno D, Angiolillo DJ. Pre-Treatment With Oral P2Y 12 Inhibitors in Acute Coronary Syndromes Without ST-Segment Elevation: The Saga Continues. J Am Coll Cardiol 2019;73:915-8.
- Schüpke S, Neumann FJ, Menichelli M, et al. Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes. N Engl J Med 2019;381:1524-34.
- Tarantini G, Mojoli M, Varbella F, et al. Downstream or upstream administration of P2Y12 receptor blockers in non-ST elevated acute coronary syndromes: study protocol for a randomized controlled trial. Trials 2020;21:966.
- Tarantini G, Mojoli M, Varbella F, et al. Timing of Oral P2Y 12 Inhibitor Administration in Patients With Non-ST-Segment Elevation Acute Coronary Syndrome. J Am Coll Cardiol 2020;76:2450-9.
Clinical Topics: Acute Coronary Syndromes, Cardiac Surgery, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Prevention, Aortic Surgery, Cardiac Surgery and Arrhythmias, Interventions and ACS, Interventions and Imaging, Angiography, Nuclear Imaging
Keywords: Acute Coronary Syndrome, Purinergic P2Y Receptor Antagonists, Platelet Aggregation Inhibitors, Fibrinolytic Agents, Percutaneous Coronary Intervention, Secondary Prevention, Aspirin, Myocardial Infarction, Random Allocation, Coronary Angiography, Control Groups, Confidence Intervals, Numbers Needed To Treat, Angina, Unstable, Coronary Artery Bypass, Stroke, Pharmaceutical Preparations, Treatment Outcome, Thrombolytic Therapy, Reference Standards
< Back to Listings