Utility of Hemodynamic Profiling Using Pulmonary Artery Catheters in Cardiogenic Shock
Quick Takes
- In a large, multicenter cardiogenic shock registry, complete hemodynamic assessment using pulmonary artery catheters prior to MCS is associated with lower in-hospital mortality compared with incomplete or no assessment.
- Future studies should focus on the underlying mechanisms of how hemodynamic information might guide the timing, selection, management, and weaning of temporary MCS.
Introduction
Cardiogenic shock (CS) is an acute, heterogenous, and highly complex condition with significant mortality and morbidity.1 There has been relatively little high-quality evidence to guide the management of CS, especially with regards to the use of mechanical circulatory support (MCS) devices. Pulmonary artery catheters (PAC), once ubiquitous in the critical care setting, declined in utilization after large, randomized control trials (RCT) failed to show benefits.2,3 However, the benefits of PAC monitoring in CS remain uncertain, as the ESCAPE trial excluded CS patients and recent retrospective, nonrandomized studies have shown some promising results.4,5
Study Synopsis
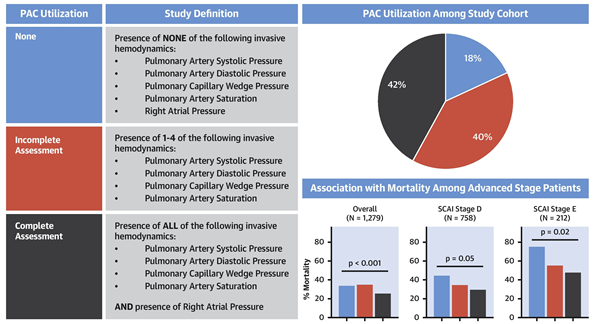
Within this context, a recent study from the Cardiogenic Shock Working Group (CSWG) examined the association between hemodynamic monitoring by PAC and in-hospital mortality in patients with CS.6 The CSWG is a growing consortium of academic and non-academic medical centers dedicated to CS research. As part of CSWG's first-phase retrospective registry, the study included 1,414 CS patients from eight centers that enrolled more than 100 patients each. CS was defined by clinical parameters and physician adjudicated. Decompensated heart failure (HF) and acute myocardial infarction (MI) were the two predominant CS etiologies, and the majority of the patients (84%) received some form of temporary MCS. The authors separated PAC usage into three groups (complete assessment, incomplete assessment, and none) and complete PAC assessment was defined as having all the following measurements: right atrial pressure, pulmonary artery systolic and diastolic pressures, pulmonary capillary wedge pressure, and pulmonary artery saturation. The study found that complete PAC assessment prior to MCS initiation was associated with the lowest in-hospital mortality compared with both incomplete and no PAC assessment. The mortality difference was seen in the overall cohort and in each of the Society for Cardiovascular Angiography and Interventions (SCAI) Stage sub-cohort (Figure 1).7
Figure 1
Role of PAC in Cardiogenic Shock Management
This study lends support to the utilization of PAC in CS by showing a 'dose-dependent' survival response based on the completeness of hemodynamic assessment by PAC and by demonstrating a consistent signal across SCAI stages and CS etiologies in a large multi-center registry. However, the results are still hypothesis-generating as there were important methodological and statistical limitations. Perhaps the most significant is that the study only examined PAC assessment prior to MCS initiation. Patients could have had complete hemodynamic assessment with PAC placed at or after MCS initiation and would have been categorized as having no assessment. Typically, one would expect PAC use to be associated with sicker patients and thus worse outcomes, but in this study PAC use was most frequent in healthier patients (no MCS, SCAI stage B) and least frequent in the sickest patients (ECMO, SCAI stage E). The most likely explanation is that higher acuity CS patients proceeded directly to MCS initiation before PAC placement first or were transferred to the study hospitals already on MCS (also categorized as no assessment in the study). This selection bias could have led to the no assessment group having more acute patients and thus worse survival even if PAC use had no real impact on survival.
In addition to the above study, there are two other non-randomized studies that have shown an association between PAC use and improved outcomes in CS. One study showed that in CS patients supported by Impella, those with PAC had better survival.4 The other study analyzed the National Inpatient Sample database and found that in decompensated HF patients, PAC use was associated with worse outcomes. However, in CS patients, PAC use was associated with improved survival compared to no PAC use (38% vs. 30%).5
While the above studies suggest that PAC use may be beneficial in CS, it is important to remember that PAC is a diagnostic tool that by itself cannot improve a patient's condition. Thus, the focus must not be whether PAC use is associated with better outcomes in CS, but how to translate the hemodynamic information obtained from PAC into appropriate interventions that lead to better outcomes. For this question, there are several potential mechanisms. Hemodynamic monitoring by PAC could enable earlier detection of clinical deterioration and more expeditious escalation of therapy and/or identification of appropriate level of MCS. PAC data could also help uncover right ventricular failure and identify patients that might need biventricular support. During treatment, PAC monitoring could provide early assessment of response to medical and MCS therapy. Lastly, as patients recover, PAC monitoring could assist in the safe weaning of MCS therapy.8
To fully realize the benefits of PAC in CS, we need to further elucidate the above mechanisms with targeted studies including RCTs where appropriate. For example, structured CS algorithms incorporating hemodynamic data from PAC have shown promising results in early studies.9,10 More research is needed on whether hemodynamic profiling via PAC can aid in the timing and optimal selection of temporary MCS. It is also increasingly recognized that CS is a heterogenous condition, and a one-size-fits-all treatment approach likely will not work. Similar to heart failure with preserved ejection fraction, phenotyping, metabotyping, and genotyping are urgently needed to gain a better understanding of the subgroups of CS. Another recent study from the CSWG applied machine learning cluster analysis to hemodynamic and metabolic variables in the same cohort of CS patients and identified three subgroups with distinct phenotypic and risk profiles.11 Future studies are needed to examine whether the various phenotypic subgroups of CS respond differently to different types of CS treatments.
In conclusion, PAC usage in CS is being revitalized by recent positive analyses of large registry and administrative databases. To realize the full potential of PAC, more research is urgently needed on the mechanisms translating hemodynamic information into optimal treatment and to better understand the heterogeneity of CS. Hemodynamic profiling by PAC has promise to be a cornerstone towards individualized, mechanistically based treatment strategies for CS.
References
- van Diepen S, Katz JN, Albert NM, et al. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation 2017;136:e232-e268.
- Connors AF Jr, Speroff T, Dawson NV, et al. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA 1996;276:889-97.
- Marik PE. Obituary: pulmonary artery catheter 1970 to 2013. Ann Intensive Care 2013;3:38.
- O'Neill WW, Grines C, Schreiber T, et al. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. Am Heart J 2018;202:33-38.
- Hernandez GA, Lemor A, Blumer V, et al. Trends in utilization and outcomes of pulmonary artery catheterization in heart failure with and without cardiogenic shock. J Card Fail 2019;25:364-71.
- Garan AR, Kanwar M, Thayer KL, et al. Complete hemodynamic profiling with pulmonary artery catheters in cardiogenic shock is associated with lower in-hospital mortality. JACC Heart Fail 2020;8:903-13.
- Baran DA, Grines CL, Bailey S, et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: this document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter Cardiovasc Interv 2019;94:29-37.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147-239.
- Basir MB, Kapur NK, Patel K, et al. Improved outcomes associated with the use of shock protocols: updates from the National Cardiogenic Shock Initiative. Catheter Cardiovasc Interv 2019;93:1173-83.
- Tehrani BN, Truesdell AG, Sherwood MW, et al. Standardized team-based care for cardiogenic shock. J Am Coll Cardiol 2019;73:1659-69.
- Thayer K, Zweck E, Ayouty M, et al. Phenoprofiling cardiogenic shock: a report from the Cardiogenic Shock Working Group. J Am Coll Cardiol 2020;75:3674-74.
Clinical Topics: Arrhythmias and Clinical EP, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Acute Heart Failure, Interventions and Imaging, Angiography, Nuclear Imaging
Keywords: Heart Failure, Shock, Cardiogenic, Pulmonary Wedge Pressure, Hospital Mortality, Atrial Pressure, Pulmonary Artery, Retrospective Studies, Blood Pressure, Selection Bias, Inpatients, Extracorporeal Membrane Oxygenation, Genotype, Stroke Volume, Registries, Morbidity, Cluster Analysis, Angiography, Critical Care, Catheters, Hospitals
< Back to Listings