2020 AHA/ACC Hypertrophic Cardiomyopathy Guideline: Contemporary Management Strategies
Quick Takes
- Clarify current risk stratification of SD in HCM.
- Understand role of CMR in HCM evaluation.
- Clarify role of invasive septal reduction treatments in obstructive HCM.
The application of contemporary cardiovascular treatments and management strategies to hypertrophic cardiomyopathy (HCM) over the last decade have altered the natural history and course of this genetic heart disease, now providing the vast majority of at-risk HCM patients the reasonable expectation for extended (if not normal) longevity and excellent quality of life.1,2 The recently updated 2020 ACC/AHA guideline for the diagnosis and treatment of HCM provides a comprehensive summary of these contemporary management strategies to provide an essential framework for which the practicing community can reliably implement these recommendations to achieve excellent outcomes for all HCM patients.2 A number of these major guideline based treatment recommendations are discussed.
For over 50 years, cardiac imaging has played a central role in the diagnosis and management of HCM.3 While echocardiography continues to be the foundation for evaluation of HCM patients,3 the current guidelines emphasize the importance of a multimodality cardiovascular imaging strategy by integrating cardiovascular magnetic resonance (CMR) imaging into the assessment of nearly all HCM patients.2,4 With high spatial resolution and the unique opportunity to detect myocardial fibrosis, CMR provides detailed characterization of the HCM phenotype to aid in diagnosis, comprehensive phenotyping and reliable identification of high-risk morphologic features such as left ventricular (LV) apical aneurysm and extent of late gadolinium enhancement (LGE) (i.e., fibrosis) for sudden death risk assessment.4-6 CMR may also inform choice of septal reduction therapy by defining unique aspects of LV anatomy that may be contributing to outflow obstruction.7
Echocardiography most reliably defines an HCM patient's hemodynamic category (nonobstructive vs. rest/provocable obstruction).8 For patients without obstruction at rest (≤50 mmHg), stress (exercise) echocardiography should be performed since certain treatment options which can substantially improve patients symptoms and outcome (e.g., disopyramide and septal reduction therapy) are not available to nonobstructive patients.2,8 For patients in whom severity of heart failure symptoms due to outflow obstruction remains ambiguous after clinical history taking, cardiopulmonary exercise testing can provide objective information on functional limitation which could inform treatment recommendations.9
For obstructive HCM patients who remain limited by heart failure (class III or IV) despite drug therapy, septal reduction therapy with surgical myectomy or alcohol septal ablation are firmly established procedures that should be offered to patients to restore normal (or near normal) quality of life.10,11 For patients who are candidates for septal reduction, strong consideration should be given to referral to comprehensive HCM centers who can perform these procedures with excellent outcome and low risk.12 Indeed, given how effective and safe these procedures have now become, it is now reasonable to consider surgical myectomy earlier in their clinical course of select obstructive HCM patients (class II) to potentially enhance natural history,2 particularly since the hemodynamic benefits of surgery may also mitigate other adverse disease related complications such as atrial fibrillation and pulmonary hypertension.13,14 It is also acceptable for patients with advanced symptoms (particularly those who are young) to consider earlier myectomy as an alternative to escalating drug therapy, after engaging in a shared decision-making discussion about pros and cons of treatment options.2
Management of severely symptomatic nonobstructive HCM patients with normal ejection fraction remains challenging.15 Beta-blocker and calcium channel blockers are preferable and if exertional dyspnea persists, oral diuretic can be used.2,15 For those nonobstructive patients who develop systolic dysfunction (EF <50%), traditional heart failure drug therapy should be considered, as well as cardiac resynchronization therapy in those with persistent advanced symptoms despite medications and a left bundle branch block (LBBB).16
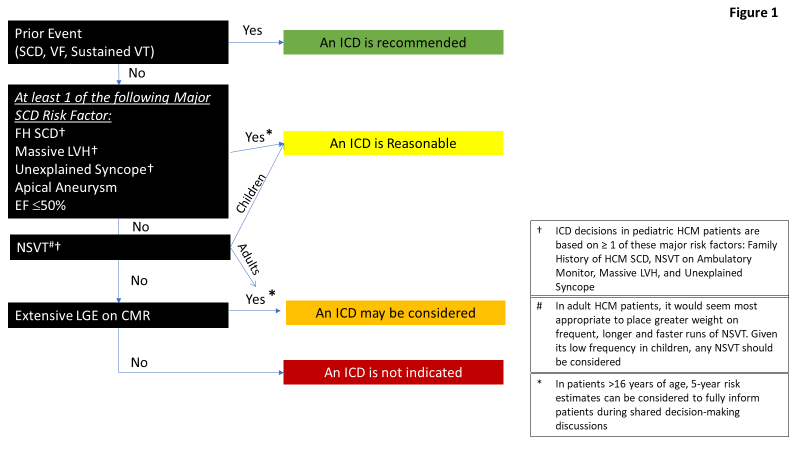
Sudden cardiac death (SCD) remains a devastating consequence of HCM, placing great weight on the importance of accurate selection of high-risk patients for implantable defibrillator (ICD) device therapy.1,2 In this regard, the guidelines continue to underscore the importance of a mature risk stratification stratify that relies on the identification of a number of noninvasive major risk markers in the clinical profile of an individual patient.2 A recommendation for primary prevention ICD therapy is reasonable to consider for HCM patients with greater than or equal to one risk marker (Figure 1). Of note, compared to prior HCM guidelines, a number of novel sudden death risk markers have now been incorporated into the routine risk stratification strategy, including LV apical aneurysm, systolic dysfunction (EF <50%) and extensive LGE5,6,17 (Figure 1). To potentially aid in ICD decision-making, quantification of an individual patient's short-term risk can also be considered using available risk tools.18 The guidelines emphasize the importance of shared decision-making, a narrative particularly relevant to helping resolve complex HCM management decisions which inevitably arise as part of this complex, heterogeneous disease.2
Figure 1: Identification of High-Risk HCM Patients for ICD
EF = ejection fraction; CMR = cardiovascular magnetic resonance; FH = family history; ICD = implantable cardioverter defibrillator; LGE = late gadolinium enhancement; LVH = left ventricular hypertrophy; NSVT = nonsustained ventricular tachycardia; SCD = sudden cardiac death; VF = ventricular fibrillation; VT = ventricular tachycardia
Atrial fibrillation (AF) in HCM is associated with an increased risk for embolic stroke and therefore prophylactic anticoagulation is recommended for paroxysmal or chronic AF,19 independent of the CHA2DS2VASc after taking into specific consideration for individual patients such as life-style modifications, risk of hemorrhagic complications, and expected compliance.2
Mild to moderate levels of recreational level physical exercise continue to be supported as important for overall cardiovascular health in patients with HCM, with no evidence that this level of physical activity increases risk in the vast majority of HCM patients.20 HCM patients who are interested in engaging in more intense levels of physical exercise should understand that risk for sudden death may be increased21 and would benefit from evaluation by an expert HCM team in order to assess overall risk benefits.
Given the autosomal dominant transmission of HCM, patients should be counseled regarding genetic transmission of the disease and family members should undergo screening.2,22 Using cardiovascular imaging, evaluation of at-risk family members can begin at any time but no later than the beginning of puberty and should be repeated every 1-2 years until the end of adolescence and then if imaging remains normal every 3 to 5 years.2 Alternatively, genetic testing strategy can also be considered but is predicated on the identification of a pathogenic mutation in the proband, which occurs in approximately 50% of HCM patients.22 The guidelines emphasize the importance of reconfirming reported pathogenicity of variants every 2 to 3 years since variants classification can be upgraded or downgraded with emerging evidence, impacting recommendations for continued surveillance of family members.23
References
- Maron BJ, Rowin EJ, Casey SA, Maron MS. How hypertrophic cardiomyopathy became a contemporary treatable genetic disease with low mortality: shaped by 50 years of clinical research and practice. JAMA Cardiol 2016;1:98-105.
- Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2020;76:e159-e240.
- Maron BJ, Maron MS. The remarkable 50 years of imaging in HCM and how it has changed diagnosis and management: from M-mode echocardiography to CMR. JACC Cardiovasc Imaging 2016;9:858-72.
- Maron MS, Rowin EJ, Maron BJ. How to image hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 2017;10:e005372.
- Rowin EJ, Maron BJ, Haas TS, et al. Hypertrophic cardiomyopathy with left ventricular apical aneurysm: implications for risk stratification and management. J Am Coll Cardiol 2017;69:761-73.
- Weng Z, Yao J, Chan RH, et al. Prognostic value of LGE-CMR in HCM: a meta-analysis. JACC Cardiovasc Imaging 2016;9:1392-1402.
- Patel P, Dhillon A, Popovic ZB, et al. Left ventricular outflow tract obstruction in hypertrophic cardiomyopathy patients without severe septal hypertrophy: implications of mitral valve and papillary muscle abnormalities assessed using cardiac magnetic resonance and echocardiography. Circ Cardiovasc Imaging 2015;8:e003132.
- Rowin EJ, Maron BJ, Olivotto I, Maron MS. Role of exercise testing in hypertrophic cardiomyopathy. JACC Cardiovasc Imaging 2017;10:1374-86.
- Coats CJ, Rantell K, Bartnik A, et al. Cardiopulmonary exercise testing and prognosis in hypertrophic cardiomyopathy. Circ Heart Fail 2015;8:1022-31.
- Kotkar KD, Said SM, Dearani JA, Schaff HV. Hypertrophic obstructive cardiomyopathy: the Mayo experience. Ann Cardiothorac Surg 2017;6:329-36.
- Batzner A, Pfeiffer B, Neugebauer A, Aicha D, Blank C, Seggewiss H. Survival after alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol 2018;72:3087-94.
- Maron BJ, Dearani JA, Ommen SR, et al. Low operative mortality achieved with surgical septal myectomy: role of dedicated hypertrophic cardiomyopathy centers in the management of dynamic subaortic obstruction. J Am Coll Cardiol 2015;66:1307-08.
- Geske JB, Konecny T, Ommen SR, et al. Surgical myectomy improves pulmonary hypertension in obstructive hypertrophic cardiomyopathy. Eur Heart J 2014;35:2032-9.
- Boll G, Rowin EJ, Maron BJ, Wang W, Rastegar H, Maron MS. Efficacy of combined Cox-Maze IV and ventricular septal myectomy for treatment of atrial fibrillation in patients with obstructive hypertrophic cardiomyopathy. Am J Cardiol 2020;125:120-26.
- Maron MS, Rowin EJ, Olivotto I, et al. Contemporary natural history and management of nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2016;67:1399-1409.
- Cappelli F, Morini S, Pieragnoli P, et al. Cardiac resynchronization therapy for end-stage hypertrophic cardiomyopathy: the need for disease specific eligibility criteria. J Am Coll Cardiol 2018;71:464-66.
- Maron MS, Rowin EJ, Wessler BS, et al. Enhanced American College of Cardiology/American Heart Association strategy for prevention of sudden cardiac death in high-risk patients with hypertrophic cardiomyopathy. JAMA Cardiol 2019;4:644-57.
- O'Mahony C, Jichi F, Ommen SR, et al. International external validation study of the 2014 European Society of Cardiology guidelines on sudden cardiac death prevention in hypertrophic cardiomyopathy (EVIDENCE-HCM). Circulation 2018;137:1015-23.
- Rowin EJ, Hausvater A, Link MS, et al. Clinical profile and consequences of atrial fibrillation in hypertrophic cardiomyopathy. Circulation 2017;136;2420-36.
- Saberi S, Wheeler M, Bragg-Gresham J, et al. Effect of moderate-intensity exercise training on peak oxygen consumption in patients with hypertrophic cardiomyopathy: a randomized clinical trial. JAMA 2017;317:1349-57.
- Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation 2009;119:1085-92.
- Burke MA, Cook SA, Seidman JG, Seidman CE. Clinical and mechanistic insights into the genetics of cardiomyopathy. J Am Coll Cardiol 2016;68:2871-86.
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405-23.
Clinical Topics: Anticoagulation Management, Arrhythmias and Clinical EP, Diabetes and Cardiometabolic Disease, Heart Failure and Cardiomyopathies, Noninvasive Imaging, Prevention, Pulmonary Hypertension and Venous Thromboembolism, Anticoagulation Management and Atrial Fibrillation, Implantable Devices, EP Basic Science, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Acute Heart Failure, Pulmonary Hypertension, Echocardiography/Ultrasound, Exercise, Hypertension
Keywords: Heart Failure, Disopyramide, Gadolinium, Quality of Life, Calcium Channel Blockers, Cardiac Resynchronization Therapy, Atrial Fibrillation, Defibrillators, Implantable, Bundle-Branch Block, Diuretics, Hypertension, Pulmonary, Exercise Test, Longevity, Stroke Volume, Virulence, Decision Making, Cardiomyopathy, Hypertrophic, Death, Sudden, Cardiac, Echocardiography, Exercise, Risk Assessment, Dyspnea, Aneurysm, Primary Prevention, Fibrosis, Genetic Testing, Phenotype, Mutation, Magnetic Resonance Spectroscopy, Anticoagulants, Medical History Taking
< Back to Listings