Worshipping at the Altar of Evidence-Based Medicine: Should Clinicians Wait for Outcomes Data Before Prescribing?
Quick Takes
- How to place into context use of novel agents in the absence of cardiovascular outcomes data.
- What are the components of complex decision-making in clinical situations in the absence of cardiovascular outcomes data.
For so long, we have all worshipped at the altar of evidence-based medicine that prescribing a medication without having compelling cardiovascular outcomes data to support its use would be considered blasphemy. It is not entirely surprising that we have set the bar so high. After all, a drug can be safe, can follow scientific principles and yet not deliver on its expected outcome due to unexpected "off-target" effects.
The story of the CETP (cholesteryl ester transfer protein) inhibitors is the perfect example. The development of the drug was motivated by the observation that those with genetic CETP deficiency have markedly elevated high-density lipoprotein-cholesterol (HDL-C) level and reduced levels of low-density lipoprotein-cholesterol (LDL-C). Yet, despite the scientific principles, the expected results did not play out in the first large trial of CETP inhibitors. In this trial, ILLUMINATE, the effects of the CETP inhibitor torcetrapib were studied in a randomized fashion and the trial was terminated early due to an increase in cardiovascular events and death in the treatment group compared with placebo.1 The authors concluded that the mechanism of increased death may be due to "off-target" effects of the medication on serum blood pressure and electrolytes. Indeed, in a post-hoc analysis, it appeared that torcetrapib-treated patients were at greatest risk of death if the drop in potassium or rise in bicarbonate levels was greater than the median change.2
Despite the best laid plans then, some medications never deliver on their promise in the outcomes-based trials. In the face of increasing costs for clinical trials, more operational challenges of large-scale randomized controlled trials and limitations imposed by unexpected circumstances, such as unexpected global pandemics, clinicians have been recently challenged to ask themselves whether we should still "worship" the god of evidence-based medicine in the same way.
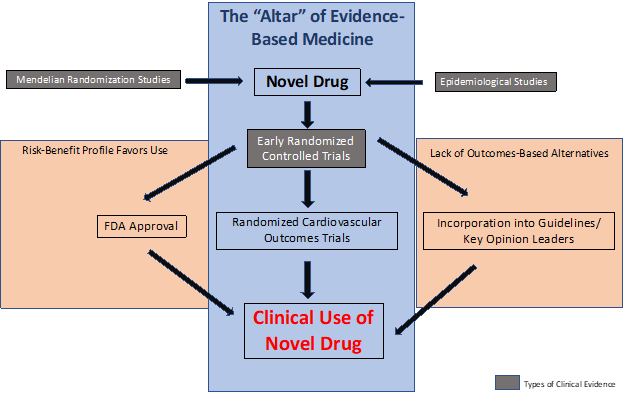
We must ask ourselves what level of "evidence" or "expert opinion" do we need before we can adopt a drug into our clinical practice. What value do observational and Mendelian randomization studies add? When it comes to interpretation of the data, is the US Food and Drug Administration (FDA) approval enough? Or do we also need expert endorsements, key opinion leaders to "buy in" and guidelines committees to include the drug into the guidelines? Or is the holy grail of the randomized controlled trial with benefit for outcomes data absolutely necessary for us to start incorporating the medication into the care of our patients (Figure 1)?
The answer? In my opinion... it depends!
Figure 1
Stuck Between a Rock and a Hard Place
Perhaps the biggest lesson to be learned from the COVID-19 pandemic is that when the disease is winning, we must make difficult choices. As time goes on, we can refine these choices, but when presented with two bad options, clinicians must sometimes lean into the option that is less undesirable if neither option is perfect. For diseases in which we don't have any other treatment options or if the patient would die without additional therapy, this decision-making is straightforward. In such cases, the use of an approved FDA drug can be obtained for off-label use, emergency use authorization or in a "compassionate use" capacity if the drug is still under investigation but no comparable therapies are available. The decision-making becomes more complex when the risk of leaving a patient untreated does not result in his/her immediate death but increases risk of adverse outcomes or death many years later or when there is a lack of therapeutic alternatives. That risk must be balanced against the risk of using a drug while awaiting cardiovascular outcomes data to support its clinical use. The metric for decision-making becomes even more complicated when it comes to prevention, rather than treatment, of disease.
This is the story of bempedoic acid (Nexletol®) and the combination tablet of bempedoic acid and ezetimibe (Nexlizet™) in patients with known atherosclerotic cardiovascular disease (ASCVD). Bempedoic acid is a novel ATP-citrate lyase inhibitor (ACL inhibitor) that works two steps upstream of statins in the cholesterol synthesis pathway and has been shown to lower LDL-C by 17-19%3 alone and by 38%4 when used in combination with ezetimibe. The drug was approved by the FDA for clinical use in February 2020 and given that its biological action is in the same pathways as statins, many of its clinical effects in early clinical trials were similar to those of statins.3,4 Based on its efficacy and safety profile, the clinical use of bempedoic acid was subsequently incorporated into the 2020 Consensus Statement by the American Association of Clinical Endocrinology (AACE) even in the absence of outcomes data.5 The CLEAR-Outcomes trial, which is now fully enrolled with >14,000 patients, is a large randomized controlled event-driven trial of bempedoic acid versus placebo for risk reduction of cardiovascular outcomes and is expected to be completed in 2022.
There are many levels of evidence in clinical medicine we must consider: randomized controlled trials, Mendelian randomization studies, and epidemiological data. The question of whether all three types of evidence are necessary or two out of three are enough to establish a causal relationship and change clinical behavior often emerges. The Mendelian randomization studies and epidemiological data are powerful types of evidence but fall outside the altar of evidence-based medicine (Figure 1). So, is two out of three enough or do we absolutely need the randomized outcomes trials to make conclusions?
In the case of LDL-C lowering and coronary heart disease (CHD) death, all three types of evidence are available and strongly support the linear risk relationship between these LDL-C and CHD risk, both in primary and secondary prevention. However, despite knowing the preventive intervention that is necessary (i.e., LDL-C lowering) in clinical practice, 7 out of 10 patients are not at their LDL-C goals.6 There are many reasons for this, and many of these patients are unable to achieve their goals due to statin intolerance or statin side effects. Furthermore, many patients require "combination" therapy with statin as well as non-statin agents to adequately lower their LDL-C to the achieved goal. To this end, we have all encountered patients with established ASCVD who are on maximally tolerated statin, ezetimibe and PCSK9 inhibitor (all drugs with outcomes data to support their use) and yet have not achieved their LDL-C targets or are having events while on these evidence-based medications. In such situations, we as clinicians are left to decide how to manage these patients, who we know are above their LDL-C goals and need imminent additional LDL-C lowering to reduce their CHD risk. Our options are to leave them untreated while awaiting randomized controlled trial outcomes data or to use a novel LDL-C lowering drug that is FDA approved and endorsed by professional organizations but does not yet have outcomes data. So, are we doing our patients a service or disservice by not prescribing this drug while awaiting the cardiovascular outcomes trials?
Regulatory Approval and Expert Opinion Lowers the Threshold for Use
The FDA's approval is a regulatory process that incorporates several factors into its complex decision-making, including:7
- Analysis of the target condition and available treatments
- Assessment of benefits and risks from clinical data
- Strategies for managing risk
Once a drug has undergone the rigorous FDA review process, it has met minimal safety regulatory requirements, making the threshold for its use in clinical practice much lower. However, it seems that the treating clinician must also assess these components of risk assessment iteratively in his/her daily decision making when deciding whether to prescribe a drug prior to the availability of cardiovascular outcomes data. Conversely, one must be careful not to fall victim to decisions driven largely by clinical inertia (and not data) which may be driving our use of a medication in our clinical practice (i.e., fibrates for hypertriglyceridemia). The bottom line is... we cannot get stuck in our practice patterns! We must remain flexible and adaptable and constantly evaluate new and emerging data (of all types) when making decisions about which medications to prescribe our primary and secondary prevention patients. Completely limiting the use of FDA-approved and guideline-endorsed drugs while awaiting outcomes results may deprive patients of therapies that can help them achieve their LDL-C goals and potentially lower their risk. However, if you are going to worship outside the "evidence-based altar of clinical medicine", careful patient selection, consideration of alternatives and shared and collective decision making (which includes a careful clinician-patient discussion regarding risks and benefits) with the patient are critical steps to avoid committing blasphemy.
References
- Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007;357:2109-22.
- ILLUMINATE: use of torcetrapib increases risk of cardiovascular disease. Nat Rev Cardiol 2008;5:122.
- Goldberg AC, Leiter LA, Stroes ESG, et al. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR Wisdom randomized clinical trial. JAMA 2019;322:1780–88.
- Ballantyne CM, Laufs U, Ray KK, et al. Bempedoic acid plus ezetimibe fixed-dose combination in patients with hypercholesterolemia and high CVD risk treated with maximally tolerated statin therapy. Eur J Prev Cardiol 2020;27:593-603.
- Garber AJ, Handelsman Y, Grunberger G, et al. CONSENSUS STATEMENT BY THE AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY ON THE COMPREHENSIVE TYPE 2 DIABETES MANAGEMENT ALGORITHM - 2020 EXECUTIVE SUMMARY. Endocr Pract 2020;26:107-39.
- Wong ND, Young D, Zhao Y, et al. Prevalence of the American College of Cardiology/American Heart Association statin eligibility groups, statin use, and low-density lipoprotein cholesterol control in US adults using the National Health and Nutrition Examination Survey 2011-2012. J Clin Lipidol 2016;10:1109-18.
- Development and Approval Process (FDA website). 2019. Available at: https://www.fda.gov/drugs/development-approval-process-drugs . Accessed 3/28/2021.
Clinical Topics: Diabetes and Cardiometabolic Disease, Dyslipidemia, Prevention, Lipid Metabolism, Nonstatins, Novel Agents
Keywords: Primary Prevention, Cholesterol, LDL, Cholesterol Ester Transfer Proteins, Cholesterol, HDL, Bicarbonates, Blood Pressure, Pharmaceutical Preparations, Potassium, United States Food and Drug Administration, Pandemics, Pandemics, Mendelian Randomization Analysis, Cardiovascular Diseases, Evidence-Based Medicine, Decision Making
< Back to Listings