The Hemodynamics of Pericardial Restraint in Heart Failure
Quick Takes
- Pericardial restraint is an important contributor to left heart filling pressures in heart failure and compromises left ventricular filling.
- Decongestion and reduction in heart size alleviates pericardial restraint in heart failure with reduced ejection fraction. This contributes to the improvement in cardiac output in heart failure with decongestion despite reductions in 'filling pressures'.
- Right atrial pressure is a reliable surrogate for pericardial pressure and can be used clinically to estimate the degree of relative pericardial restraint.
Pericardial Contribution to Intracavitary Pressure
When a catheter is inserted into the left ventricle (LV), the measured end diastolic pressure is often used as a clinical estimate of ventricular preload. However, since it is the end diastolic volume that actually determines the degree of end-diastolic myocyte stretch and Frank-Starling related stroke volume response, it is really the end diastolic volume that best reflects LV preload, and not the end diastolic pressure. Therefore, the left ventricular end-diastolic pressure (LVEDP) remains an imperfect preload estimate as it represents the numerical sum of two opposing vector forces 1) a compressive contact force on the left ventricle mediated across the lateral wall and septum from the pericardium (that attempts to collapse the LV) and 2) effective distending pressure (i.e., transmural pressure) that exerts force outwards from the center of the LV (that attempts to expand the LV facilitating filling). Even in normal hearts, as much as 30 to 40% of intracavitary pressure results from outside compressive contact stress on the LV mediated across the lateral wall by the pericardium, and from the right ventricle (RV) across the interventricular septum.1
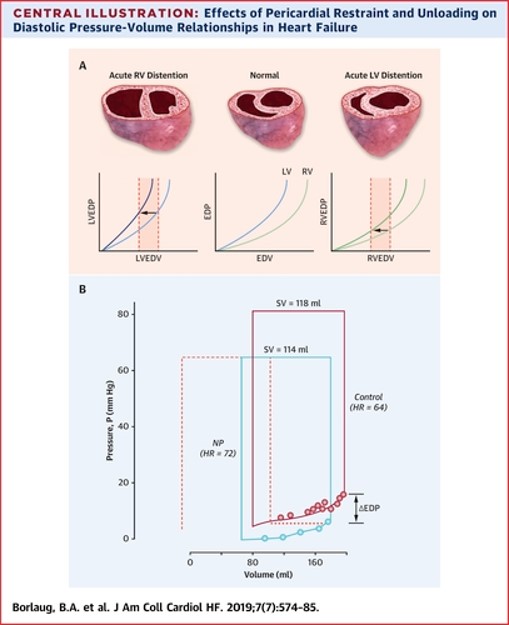
Figure 1: Effects of Pericardial Restraint and Unloading on Diastolic Pressure-Volume Relationships in Heart Failure
Pericardial Restraint and Frank Starling's Law
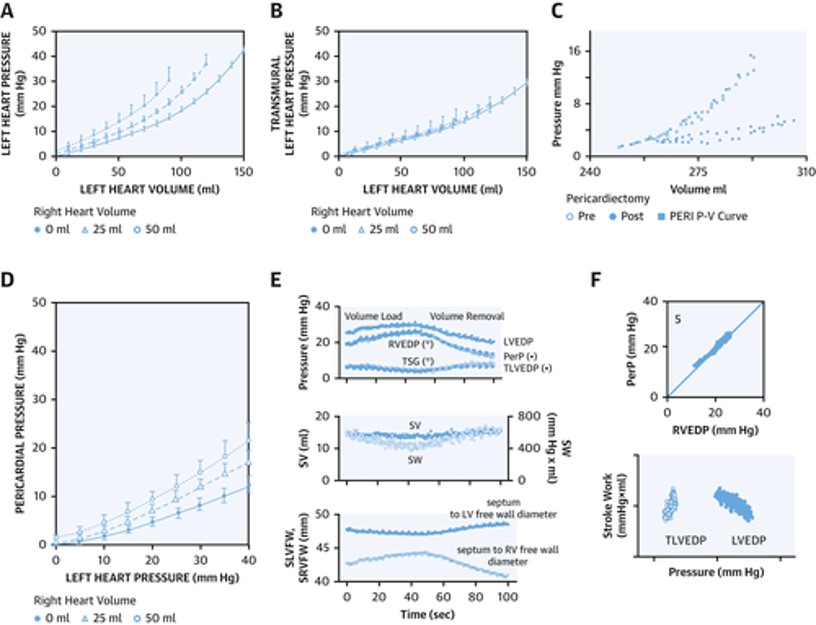
If we reflect on Frank-Starling's seminal law of the heart, it essentially implies a compensatory increase in myocardial contractile performance as the ventricular preload increase thereby maintaining cardiac homeostasis. However, due to the effects of pericardial restraint there can be an uncoupling between LVEDP and LV end diastolic volume (i.e., true preload) at higher values.2 Failure to account for pericardial restraint is the actual cause of the apparent descending limb of the Frank Starling curve relating severely increased LVEDP to worsening stroke volume (Figure 2). This apparent descending limb is actually due to worsening pericardial restraint as venous return increases to the point that the RV becomes distended, thereby increasing pericardial pressure and compromising LV preload and stroke volume despite increasing LVEDP.3
Figure 2: Hemodynamics of Pericardial Restraint and Ventricular Interaction
Measuring pericardial pressure is not practical clinically but right atrial (RA) pressure is a very reliable estimate of pericardial pressure as validated by Tyberg et al.4 Therefore, by subtracting RA pressure from the LVEDP, we obtain the LV transmural pressure (true distending pressure in the LV) which accounts for the degree of pericardial restraint. In other words, LV transmural pressure = LVEDP – RA pressure. Any increase in RA or pericardial pressure from cardiac pathology will tend to decrease LV preload for any measured LVEDP.
Contributory Factors to Relative Pericardial Restraint
An increase in pericardial pressure and enhancement of pericardial diastolic ventricular interaction can be caused by either (i) enlarged epicardial heart volume stretching a normal pericardium shifting it to a steeper portion of its pressure-volume relationship (ii) alteration of the inherent properties of the pericardium via pericardial fibrosis which can occur via radiation, aging, inflammation or obesity (which in its most extreme form causes what is currently considered constrictive pericarditis) (iii) accumulation of epicardial fat between the visceral and parietal pericardium (iv) pericardial fluid accumulation (which if clinically overt is referred to as cardiac tamponade).
Clinical Assessment of Pericardial Restraint
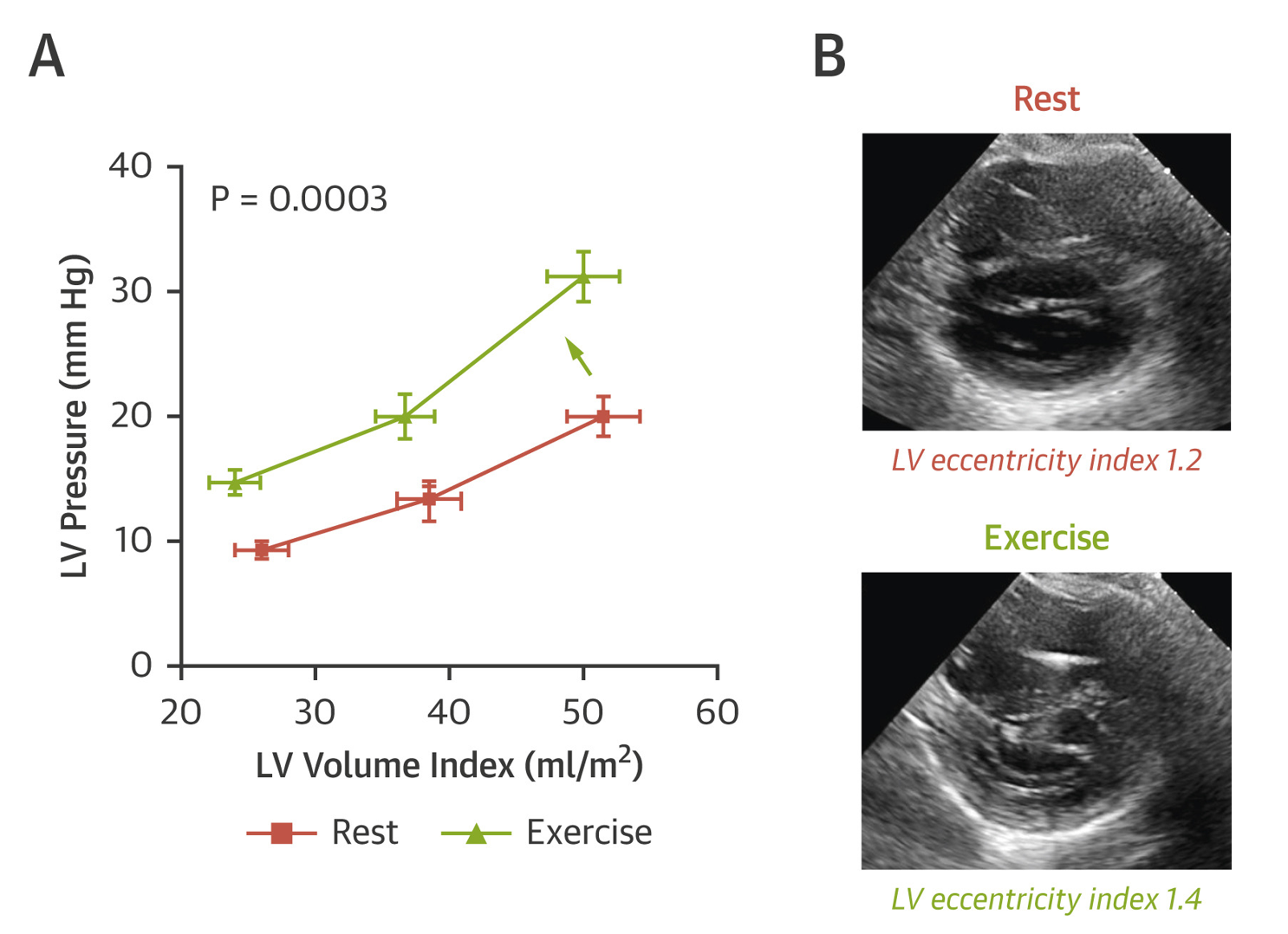
Simultaneous measurement of RA pressure and pulmonary capillary wedge pressure (PCWP), and the response of their relationship to dynamic preload stress from fluid loading or exercise can provide important information about the degree that pericardial restraint contributes to the absolute PCWP elevation. The transmural pressure (PCWP-RA pressure) best estimates true LV preload or distending forces. In other words, an increase in pericardial restraint leads to higher PCWP for any given LV preload or end diastolic volume. This effectively causes a parallel upward or leftward shift of the LV pressure-volume relationship without there being an intrinsic change in the diastolic properties of the underlying myocardium.5 The net result of such restraint is a competing effect between the RV and LV for filling, with the LV becoming increasingly "D" shaped or flattened at the expense of RV filling. This can be quantified by the eccentricity index in the short axis view, where increased pericardial restraint results in a decrease in the septolateral LV dimension from relative LV collapse. This results in an increase in eccentricity index as pericardial restraint worsens (Figure 3).
Figure 3: Obesity-Related HFpEF With Enhanced Pericardial Restraint (A)
The total epicardial volume enlargement and resultant relative pericardial restraint can be from several causes including dilated cardiomyopathy, chronic RV dilation from pulmonary arterial hypertension, acute RV dilation from RV infarction or pulmonary embolism, or severe biatrial enlargement from permanent atrial fibrillation. We are also increasingly recognizing the role of relative pericardial restraint in heart failure with preserved ejection fraction (HFpEF), particularly of the obese,6 and those with pulmonary vascular disease and abnormal RV-PA coupling.7
Using Pericardial Pressure to Understand Response to Drug Therapy in Heart Failure
Diuretics work in large part by alleviating pericardial restraint and invariably improve forward cardiac output and true LV preload after adequate decongestion and reduction in epicardial heart volumes and pericardial pressure. Nitroprusside lowers pericardial pressure8 from improved mesenteric venous compliance,9 whereas angiotensin worsened mesenteric venous compliance and increased pericardial pressure. Building on this, Konstam et al. demonstrated that part of the acute benefit of ACE inhibition (with decreasing angiotensin) was likely related to preload reduction in patients with dilated RVs that subsequently improved LV filling from relieving pericardial restraint.10 Nitroglycerin also favorably impacts filling pressures in heart failure and alleviates angina through splanchnic vasodilation and preload reduction. Impressively, the reduction in LVEDP with nitroglycerin is not typically associated with a fall in stroke volume (and often paradoxically increases stroke volume), in large part due to the relief of extrinsic restraint from right heart preload reduction with a downward shift in the LV pressure volume relationship.11 Even left ventricular pacing may exert part of its benefit through earlier LV filling during the time of lowest extrinsic restraint before RV filling and distention has worsened diastolic ventricular interaction.12
Using Understanding of Pericardial Pressure to Guide Decongestion
These experiments explain why in clinical practice, no clinical measure has proven more reliable to guide safe diuresis in heart failure than the degree of jugular venous pressure elevation (as a reflection of right atrial and therefore pericardial pressure elevation).13 As long as pericardial pressure (and therefore jugular venous pressure) is elevated, volume removal with diuresis is very unlikely to compromise cardiac output, and in most cases actually improves forward cardiac output from relief of pericardial restraint on the LV.14 This is also in part because RA pressure and PCWP tend to correlate 1:1 in most decompensated heart failure patients, due to the contribution from pericardial restraint in dilated volume overloaded hearts.15 Stevenson initially demonstrated that decompensated heart failure patients could have their PCWP lowered from a mean of 30 to 10 mmHg with no decrease in cardiac output or LV preload. In fact, stroke volume and stroke work actually improved despite marked PCWP reduction likely related to relief of pericardial restraint.14 Therefore, there is little justification to inadequate decongestion in dilated cardiomyopathy for the purpose of maintaining preload and cardiac output.
Conclusions
The normal pericardium plays a seminal role in modulating intracardiac pressures and response to drug therapy in various forms of heart failure. Understanding the relationship between pericardial, right atrial and left sided filling pressures has important clinical implications in the management of clinical heart failure.
References
- Dauterman K, Pak PH, Maughan WL, et al. Contribution of external forces to left ventricular diastolic pressure. Implications for the clinical use of the Starling law. Ann Intern Med 1995;122:737–42.
- Borlaug BA, Reddy YNV. Some laws were not made to be broken: when Frank-Starling reserve is lost in heart failure. JACC Cardiovasc Imaging 2017;10:1250–52.
- Moore TD, Frenneaux MP, Sas R, et al. Ventricular interaction and external constraint account for decreased stroke work during volume loading in CHF. Am J Physiol Heart Circ Physiol 2001;281:H2385-91.
- Tyberg JV, Taichman GC, Smith ER, Douglas NW, Smiseth OA, Keon WJ. The relationship between pericardial pressure and right atrial pressure: an intraoperative study. Circulation 1986;73:428–32.
- Janicki JS, Weber KT. The pericardium and ventricular interaction, distensibility, and function. Am J Physiol 1980;238:H494-503.
- Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6–19.
- Gorter TM, Obokata M, Reddy YNV, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J 2018;39:2825–35.
- Carroll JD, Lang RM, Neumann AL, Borow KM, Rajfer SI. The differential effects of positive inotropic and vasodilator therapy on diastolic properties in patients with congestive cardiomyopathy. Circulation 1986;74:815–25.
- Smiseth OA, Manyari DE, Lima JA, et al. Modulation of vascular capacitance by angiotensin and nitroprusside: a mechanism of changes in pericardial pressure. Circulation 1987;76:875–83.
- Konstam MA, Kronenberg MW, Udelson JE, et al. Effect of acute angiotensin converting enzyme inhibition on left ventricular filling in patients with congestive heart failure. Relation to right ventricular volumes. Circulation 1990;81:III115-122.
- Smith ER, Smiseth OA, Kingma I, Manyari D, Belenkie I, Tyberg JV. Mechanism of action of nitrates. Role of changes in venous capacitance and in the left ventricular diastolic pressure-volume relation. Am J Med 1984;76:14–21.
- Bleasdale RA, Turner MS, Mumford CE, et al. Left ventricular pacing minimizes diastolic ventricular interaction, allowing improved preload-dependent systolic performance. Circulation 2004;110:2395–400.
- From AM, Lam CSP, Pitta SR, et al. Bedside assessment of cardiac hemodynamics: the impact of noninvasive testing and examiner experience. Am J Med 2011;124:1051–7.
- Stevenson LW, Tillisch JH. Maintenance of cardiac output with normal filling pressures in patients with dilated heart failure. Circulation 1986;74:1303–8.
- Baker AE, Dani R, Smith ER, Tyberg JV, Belenkie I. Quantitative assessment of independent contributions of pericardium and septum to direct ventricular interaction. Am J Physiol 1998;275:H476-483.
Clinical Topics: Arrhythmias and Clinical EP, Heart Failure and Cardiomyopathies, Pericardial Disease, Prevention, Atrial Fibrillation/Supraventricular Arrhythmias, Acute Heart Failure
Keywords: Heart Failure, Primary Prevention, Pericarditis, Constrictive, Heart Ventricles, Stroke Volume, Cardiac Volume, Nitroglycerin, Nitroprusside, Blood Pressure, Blood Pressure, Cardiac Tamponade, Pulmonary Wedge Pressure, Atrial Fibrillation, Cardiomyopathy, Dilated, Vasodilation, Diuretics, Angiotensins, Angiotensin-Converting Enzyme Inhibitors, Dilatation, Pericardium, Myocardium, Pulmonary Embolism, Diuresis, Stroke, Inflammation, Catheters, Obesity, Muscle Cells, Infarction, Homeostasis, Venous Pressure, Fibrosis
< Back to Listings