New Insights into the Complex Nature of Ischemia with No Obstructive Coronary Artery Disease
Quick Takes
- The pathophysiology of ischemia and no obstructive coronary artery disease (INOCA) is complex.
- Ischemia and angina commonly improve in INOCA, but are not necessarily correlated.
- Stress echocardiography to evaluate severity of ischemia is often not a surrogate for clinical symptom severity in patients with INOCA.
Commentary based on Reynolds HR, Picard MH, Spertus JA, et al. Natural history of patients with ischemia and no obstructive coronary artery disease: the CIAO-ISCHEMIA study. Circulation 2021;Jun 1:[Epub ahead of print].1
Introduction
Ischemia and No Obstructive Coronary Artery disease (INOCA) refers to the presence of myocardial ischemia in the absence of a ≥50% diameter stenosis.2 An increasing number of patients with INOCA are being seen, with a relatively higher prevalence in women.3,4
Although INOCA is associated with a more favorable prognosis than severe coronary artery disease (CAD), it is not benign; however, no clinical practice guidelines currently exist for its management.5 Additionally, the natural history of symptoms and ischemia in INOCA is not clearly understood. The recently published Changes in Ischemia and Angina over 1 Year Among ISCHEMIA Trial Screen Failures With no Obstructive CAD (CIAO-ISCHEMIA) study highlights the complex nature of angina and ischemia in patients with INOCA.
Aims and Rationale
The International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial investigated whether clinical outcomes in stable CAD were better with an initial invasive or conservative management strategy and ultimately found no benefit to initial revascularization compared to medical therapy alone.6 ISCHEMIA enrolled 5,179 patients with moderate to severe ischemia who were randomized to (1) an initial invasive strategy and medical therapy, or (2) an initial conservative strategy involving medical therapy and subsequent angiography if medical therapy failed.6
Based on the selection criteria, the study sample excluded a large proportion (21%) of participants with no obstructive coronary disease based on coronary computed tomography angiography (CCTA). The investigators of CIAO-ISCHEMIA addressed this gap by enrolling a portion of these excluded participants, specifically those who had demonstrated ischemic symptoms on stress echocardiography (208 participants). This study group was directly compared to participants from ISCHEMIA randomized to either arm with ≥50% stenosis on CCTA.
Methods and Scope
Interpretation of stress echocardiograms involved blinding of interpreters to participant enrollment (CIAO-ISCHEMIA or ISCHEMIA), clinical parameters, stress electrocardiogram, and CCTA results. Blinded 1-year follow-up stress echocardiograms were also performed for 194 CIAO-ISCHEMIA participants at the same interpreting laboratory, while ISCHEMIA was still enrolling participants. Stress wall motion index scores were calculated for 16 left ventricular segments.
Assessment of angina symptoms for CIAO-ISCHEMIA participants was also performed, primarily utilizing the Seattle Angina Questionnaire (SAQ)7 at the time of enrollment, at 6 months, and at 1 year. Information on clinical outcomes related to myocardial infarction, unstable angina, heart failure, stroke, resuscitated cardiac arrest, death, and chest pain hospitalization was collected over the 1-year follow-up period.
INOCA Patient Characteristics
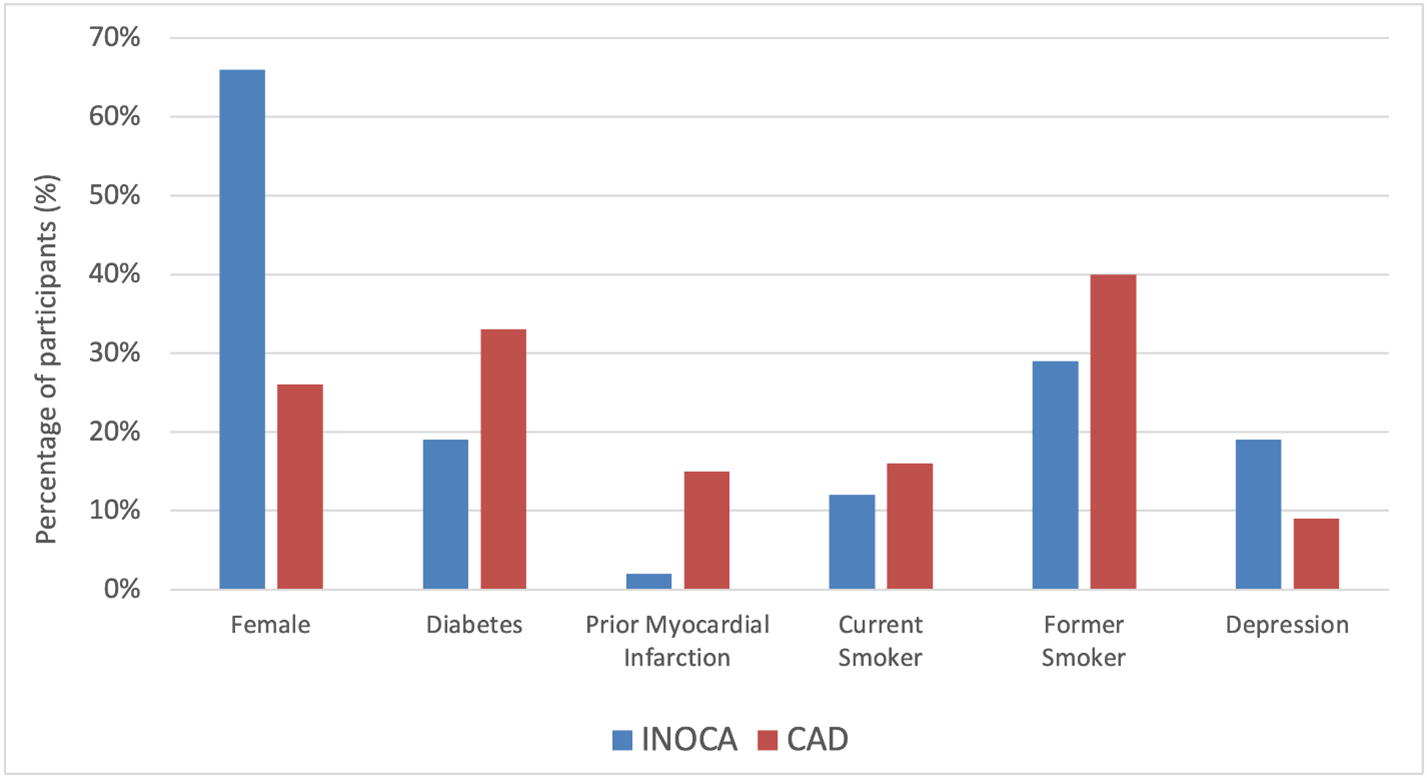
As summarized in Figure 1, compared to participants with obstructive CAD, those with INOCA were predominantly female (66% vs. 26%, p<0.001), younger (median age 63 years vs. 66 years, p=0.001), with lower rates of diabetes (19% vs. 33%, p<0.001), prior myocardial infarction (2% vs. 15%, p<0.001), current smoking (12% vs. 16%, p=0.001) and former smoking history (29% vs. 40%, p=0.001); higher rates of depression (19% vs. 9%, p<0.001) observed.
Figure 1
Stress Echocardiography in INOCA and Longitudinal Changes
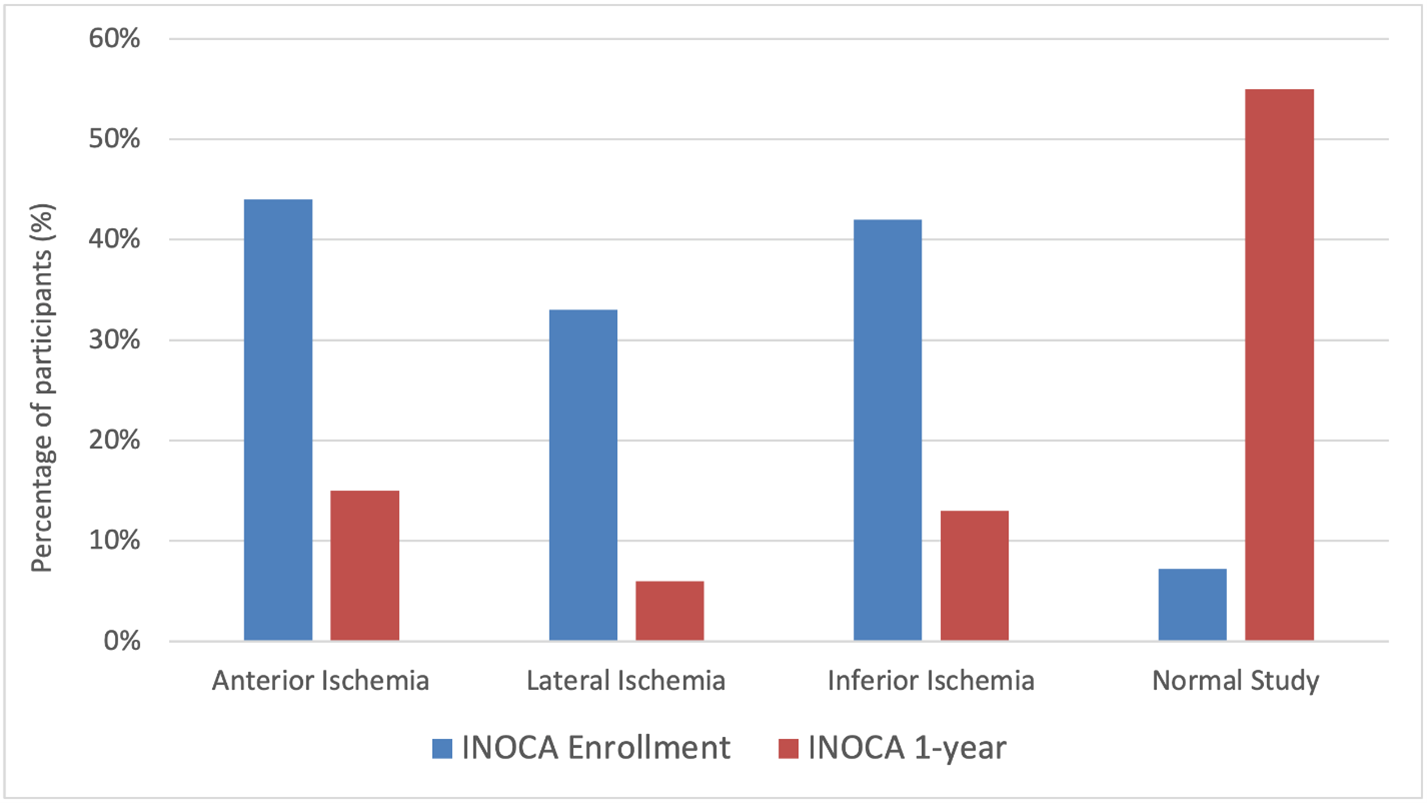
At enrollment, participants with INOCA and obstructive CAD were both noted to have a median of four ischemic segments on stress echocardiography, with anterior ischemia being more common in INOCA (44% vs. 35%, p=0.027).
At 1-year follow up, 50% of INOCA participants had normal stress echocardiograms, and the median number of ischemic segments was 0, although moderate/severe ischemia was present in 23% of participants. A reduction of at least two ischemic segments was seen in 68% of participants. Echocardiographic location of ischemia in INOCA participants at enrollment and 1-year is summarized in Figure 2. Compared to testing from enrollment, 21% of participants had abnormal echocardiograms at 1-year without improvement by at least two ischemic segments.
Figure 2
A striking feature of INOCA is its female predominance, and the pathogenesis may in part be driven by supply-demand mismatch through inadequate coronary vasodilation from microvascular dysfunction, occurring with or without concomitant atherosclerosis.8 These changes may be occurring in response to coronary spasm or increased myocardial demand.8 The improvement noted over a 1-year interval in INOCA participants may be explained by the dynamic nature of these mechanisms of disease pathogenesis.
Health Status and Angina in INOCA and Longitudinal Changes
At enrollment, participants with INOCA had a higher median SAQ summary score compared to those with obstructive CAD. At 1-year follow up, 50% of INOCA participants had ≥5 point improvement of their SAQ summary score (with 20% having ≥5 point worsening of SAQ summary score) and 43% of INOCA participants had ≥10-point improvement in the SAQ Angina Frequency sub score (with 14% having ≥10-point worsening of the SAQ Angina Frequency sub score).
Nevertheless, the results may potentially be skewed by patient-physician interactions, especially those involving reassurances considering the absence of obstructive CAD in patients with INOCA.
Correlation between Changes in Ischemia and Angina
No statistically significant difference was noted in 1-year SAQ summary score of participants with and without 1-year improvement of at least two ischemic segments (median score 92 vs. 89, p=0.56). Similarly, no statistically significant difference was noted between the groups in 1-year angina frequency. For participants with INOCA over a period of 1-year, there was no discernible correlation between change in ischemia measured by number of ischemic segments on stress echocardiography and changes in angina.
There are numerous potential contributors to the symptom of angina – physical activity, cardiac diastolic function, autonomic nervous function, and sex, to name a few – making the pathophysiology complex.9 Consequently, although it is common for ischemia and angina to improve over time in INOCA, these two entities are not necessarily linked. Based on the results of the CIAO-ISCHEMIA observational, non-randomized study, the severity of ischemia as determined by stress echocardiography cannot be used as a surrogate for severity of symptoms in patients with INOCA.
Future studies can potentially evaluate predictors of INOCA on other imaging modalities such as CCTA, in larger study samples. This would not only help improve understanding of mechanisms of ischemia in these patients but also help define practice guidelines for management of anginal symptoms in patients with INOCA.
References
- Reynolds HR, Picard MH, Spertus JA, et al. Natural history of patients with ischemia and no obstructive coronary artery disease: The CIAO-ISCHEMIA study. Circulation 2021;Jun 1:[Epub ahead of print].
- Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation 2017;135:1075-92.
- Herscovici R, Sedlak T, Wei J, Pepine CJ, Handberg E, Bairey Merz CN. Ischemia and No Obstructive Coronary Artery Disease (INOCA): what is the risk? J Am Heart Assoc 2018;7:e008868.
- Jespersen L, Hvelplund A, Abildstrom SZ, et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33:734-44.
- Tamis-Holland JE, Jneid H, Reynolds HR, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation 2019;139:e891-e908.
- Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med 2020;382:1395-1407.
- Chan PS, Jones PG, Arnold SA, Spertus JA. Development and validation of a short version of the Seattle angina questionnaire. Circ Cardiovasc Qual Outcomes 2014;7:640-47.
- Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC State-of-the-Art Review. J Am Coll Cardiol 2018;72:2625-41.
- Reynolds HR, Shaw LJ, Min JK, et al. Association of sex with severity of coronary artery disease, ischemia, and symptom burden in patients with moderate or severe ischemia: secondary analysis of the ISCHEMIA randomized clinical trial. JAMA Cardiol 2020;5:773-86.
Clinical Topics: Arrhythmias and Clinical EP, Diabetes and Cardiometabolic Disease, Dyslipidemia, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Stable Ischemic Heart Disease, Atherosclerotic Disease (CAD/PAD), Implantable Devices, SCD/Ventricular Arrhythmias, Acute Heart Failure, Interventions and Coronary Artery Disease, Interventions and Imaging, Angiography, Echocardiography/Ultrasound, Nuclear Imaging, Chronic Angina
Keywords: Dyslipidemias, Diagnostic Imaging, Angina, Stable, Coronary Artery Disease, Echocardiography, Stress, Vasodilation, Patient Selection, Constriction, Pathologic, Follow-Up Studies, Angina Pectoris, Myocardial Ischemia, Myocardial Infarction, Heart Failure, Angina, Unstable, Atherosclerosis, Angiography, Heart Arrest, Stroke, Hospitalization, Health Status, Prognosis, Electrocardiography
< Back to Listings