Can Too Much Exercise Harm The Right Ventricle?
Quick Takes
- In rare circumstances, endurance exercise may be associated with development of arrhythmogenic cardiomyopathy.
- It is likely that there is an important interaction between genetic predisposition and a facilitating effect of exercise.
- Patients with genetic mutations known to be associated with arrhythmogenic cardiomyopathies should be advised against intensive endurance exercise.
Exercise is proven to be beneficial for physical health, mental health, and longevity. In the current circumstance of a worldwide pandemic, it is also worth noting that fitness is associated with greater survival from COVID-related illness.1 Exercise is an important therapeutic tool in many chronic diseases which are associated with an unhealthy lifestyle and are unfortunately increasing in prevalence. The paradox which sees risk of cardiac death increase during acute bouts of exercise offset by a reduction in risk of death during non-exercising periods means that exercise results in a lower risk of overall death and underpins recommendations that exercise is both safe and desirable.
The human attribute of seeking to surpass and excel has resulted in greater participation in longer and more grueling sporting events. In the same way that extreme exercise may provoke musculoskeletal injury, it is important that we ask whether there are circumstances where too much exercise can be harmful for the heart. At present, both animal and human evidence suggests that high-level endurance exercise may be harmful in patients with arrhythmogenic cardiomyopathies.2 In addition, in susceptible individuals without recognized genetic abnormalities normally associated with arrhythmic cardiomyopathy, an acquired arrhythmogenic cardiomyopathy associated with endurance exercise has been described.3
Perhaps the best evidenced association between high volumes of intense exercise training and heart disease relates to atrial fibrillation (AF). A U-shaped dose-response relationship can be constructed from athletic4,5 and non-athletic population data6 describing an excess AF prevalence amongst those that are least and most active. The exact reasons for this are complex but possibly relate to some combination of enlargement of the atria,7 myocardial inflammation and scar deposition, autonomic modulation, and genetic influences. The last of these possibilities is yet to be fully interrogated but suggests that arrhythmias result from a 'double hit' phenomenon in which a genetic predisposition combines with the environmental stressors of repeated intense exercise. To date, arrhythmogenic right ventricular cardiomyopathy (ARVC) has proven the archetypal pathology in which this concept is best evidenced.
ARVC has long been recognized as an important cause of sudden cardiac death during exercise.8 Active screening for this entity and exclusion of those with an ARVC phenotype from competitive sport has been undertaken in many parts of the world. More recently the important role of exercise in driving phenotypic expression and disease progression in those individuals who carry gene mutations in the desmosomal proteins known to be associated with ARVC has been firmly established. Athletic carriers of mutations in ARVC genes undertaking endurance exercise typically develop clinical manifestations of ARVC around 10 years earlier than sedentary carriers and are more likely to develop heart failure and ventricular arrhythmias.9,10 A history of high-intensity exercise is also associated with a higher risk of arrhythmic events and right ventricular (RV) dysfunction.11,12 These clinical findings also are supported by basic research showing the development of RV dysfunction and ventricular arrhythmias in plakoglobin-deficient mice subjected to intense physical training.13 Thus, it appears prudent to advise carriers of genetic mutations known to cause ARVC against high-intensity endurance exercise.
What about those without recognized mutations in desmosomal proteins known to cause arrhythmogenic cardiomyopathies? Heidbuchel described a group of endurance athletes with unexplained RV dysfunction and malignant ventricular arrhythmias14 and later used the term "exercise-induced arrhythmogenic cardiomyopathy" to describe this clinical scenario. When genetic analysis of 47 of these athletes revealed that only six were carriers of known ARVC mutations, this suggested that exercise itself, rather than previously undiagnosed ARVC, may be causative.15 Similarly, patients with ARVC without a recognized desmosomal gene mutation (gene-elusive) are more likely to have a history of high-intensity exercise.16 We have shown that elevated troponin-I levels following ultra-endurance exercise are associated with RV dysfunction and that the RV is subjected to a greater rise in wall stress with exercise, which may provide a mechanistic link between exercise and the development of exercise-induced arrhythmogenic cardiomyopathy. In a mouse model with a mutation in the gene for plakophilin-2, phenotypic expression was not seen except in mice subjected to endurance training, providing support for the hypothesis that susceptible individuals at risk of an exercise-induced cardiomyopathy may carry a "gene-elusive" mutation, the expression of which is facilitated by exercise.
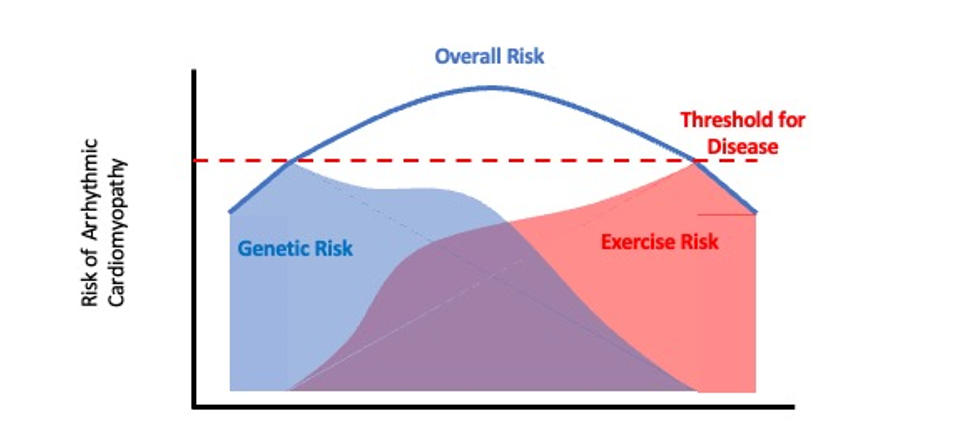
Perhaps both excessive exercise and the presence of a genetic predisposition could explain the relationship between endurance exercise and the occurrence of ventricular arrhythmias and RV dysfunction. In those with a recognized ARVC mutation, it may only take a moderate amount of exercise to hasten phenotypic expression, whereas in those "gene-elusive" individuals, who may carry a mutation which is not expressed phenotypically in sedentary individuals, high volume and/or high intensity exercise is required to trigger phenotypic and clinical expression, sometimes with tragic results. The malignancy of the genetic abnormality may determine just how much exercise is required to reach the threshold to cause disease (Figure 1). If this hypothesis is true, our challenge is now to identify the responsible genes and mechanisms to devise a strategy to identify these individuals and provide advice regarding a safe exercise prescription. After all, the aim is to safely promote good health in the general population through regular exercise.
Figure 1
References
- Christensen RAG, Arneja J, St Cyr K, Sturrock SL, Brooks JD. The association of estimated cardiorespiratory fitness with COVID-19 incidence and mortality: a cohort study. PLoS One 2021;16:e0250508.
- Prior D, La Gerche A. Exercise and arrhythmogenic right ventricular cardiomyopathy. Heart Lung Circ 2020;29:547-55.
- Heidbuchel H, Prior DL, La Gerche A. Ventricular arrhythmias associated with long-term endurance sports: what is the evidence? Br J Sports Med 2012;46:i44-50.
- Andersen K, Farahmand B, Ahlbom A, et al. Risk of arrhythmias in 52 755 long-distance cross-country skiers: a cohort study. Eur Heart J 2013;34:3624-31.
- Flannery MD, Kalman JM, Sanders P, La Gerche A. State of the Art Review: atrial fibrillation in athletes. Heart Lung Circ 2017;26:983-89.
- Elliott AD, Linz D, Mishima R, et al. Association between physical activity and risk of incident arrhythmias in 402 406 individuals: evidence from the UK Biobank cohort. Eur Heart J 2020;41:1479-86.
- Trivedi SJ, Claessen G, Stefani L, et al. Differing mechanisms of atrial fibrillation in athletes and non-athletes: alterations in atrial structure and function. Eur Heart J Cardiovasc Imaging 2020;21:1374-83.
- Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol 2003;42:1959-63.
- James CA, Bhonsale A, Tichnell C, et al. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol 2013;62:1290-97.
- Saberniak J, Hasselberg NE, Borgquist R, et al. Vigorous physical activity impairs myocardial function in patients with arrhythmogenic right ventricular cardiomyopathy and in mutation positive family members. Eur J Heart Fail 2014;16:1337-44.
- Ruiz Salas A, Barrera Cordero A, Navarro-Arce I, et al. Impact of dynamic physical exercise on high-risk definite arrhythmogenic right ventricular cardiomyopathy. J Cardiovasc Electrophysiol 2018;29:1523-29.
- Lie OH, Rootwelt-Norberg C, Dejgaard LA, et al. Prediction of life-threatening ventricular arrhythmia in patients with arrhythmogenic cardiomyopathy: a primary prevention cohort study. JACC Cardiovasc Imaging 2018;11:1377-86.
- Kirchhof P, Fabritz L, Zwiener M, et al. Age- and training-dependent development of arrhythmogenic right ventricular cardiomyopathy in heterozygous plakoglobin-deficient mice. Circulation 2006;114:1799-806.
- Heidbuchel H, Hoogsteen J, Fagard R, et al. High prevalence of right ventricular involvement in endurance athletes with ventricular arrhythmias. Role of an electrophysiologic study in risk stratification. Eur Heart J 2003;24:1473-80.
- La Gerche A, Robberecht C, Kuiperi C, et al. Lower than expected desmosomal gene mutation prevalence in endurance athletes with complex ventricular arrhythmias of right ventricular origin. Heart 2010;96:1268-74.
- Sawant AC, Bhonsale A, te Riele ASJM, et al. Exercise has a disproportionate role in the pathogenesis of arrhythmogenic right ventricular dysplasia/cardiomyopathy in patients without desmosomal mutations. J Am Heart Assoc 2014;3:e001471.
Clinical Topics: Arrhythmias and Clinical EP, Cardiovascular Care Team, COVID-19 Hub, Heart Failure and Cardiomyopathies, Sports and Exercise Cardiology, Genetic Arrhythmic Conditions, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Acute Heart Failure
Keywords: Sports, Athletes, Arrhythmogenic Right Ventricular Dysplasia, Plakophilins, Troponin I, Genetic Predisposition to Disease, gamma Catenin, Atrial Fibrillation, Prevalence, Cicatrix, Longevity, Mental Health, Pandemics, COVID-19, SARS-CoV-2, Heart Failure, Death, Sudden, Cardiac, Phenotype, Mutation, Disease Progression, Chronic Disease, Life Style, Inflammation, Prescriptions
< Back to Listings