De-Escalation of P2Y12-Inhibiting Therapies to Reduce the Risk of Bleeding After PCI
Quick Takes
- De-escalation, defined as switching from prasugrel or ticagrelor to clopidogrel as a strategy to reduce bleeding risk, commonly occurs in clinical practice.
- Routine de-escalation should not be recommended, but de-escalation is a reasonable approach in selected ACS patients.
- Based on the available evidence, an approached based platelet function testing- or genotype-guided de-escalation should be preferred, if feasible.
Dual antiplatelet therapy (DAPT) with aspirin and an oral P2Y12 receptor inhibitor (clopidogrel, prasugrel, or ticagrelor) is the cornerstone of treatment for patients with acute coronary syndrome (ACS) or undergoing percutaneous coronary intervention (PCI).1 Prasugrel and ticagrelor are characterized by more prompt and potent antiplatelet effects compared to clopidogrel, which translates into a reduction in ischemic outcomes in patients with ACS.2,3 However, this benefit is hampered by an increase in major bleeding events, underscoring the need for strategies to reduce bleeding risk without a trade-off in ischemic protection. Multiple strategies to reduce bleeding after PCI have been established through the conduct of several clinical trials. These strategies include reducing DAPT intensity (e.g., de-escalation) or shortening DAPT duration (e.g., shorten clopidogrel treatment, shorten duration of aspirin, no aspirin) (Table 1).4 In this expert analysis we will focus on providing the available evidence as well as practical recommendations on de-escalation.
Table 1: Clinical Trials Assessing Strategies to Reduce Bleeding After PCI
| De-escalation of DAPT | Shortening of DAPT duration | ||
| Shorten Clopidogrel | Shorten Aspirin | No Aspirin | |
| TOPIC | RESET | GLOBAL LEADERS | WOEST* |
| POPULAR AGE | OPTIMIZE | GLASSY | PIONEER-AF |
| TROPICAL ACS# | REDUCE ACS | STOPDAPT-2 | PCI* |
| POPULAR GENETICS # | ISAR TRIPLE* | SMART-CHOICE | RE-DUAL PCI* |
| MASTER DAPT*† | TWILIGHT | AUGUSTUS* | |
| TICO | ENTRUST-AF PCI* | ||
| MASTER DAPT*† | |||
| STOPDAPT-2 ACS | |||
* Includes patients on OAC undergoing PCI
† Shorter DAPT included both shorten clopidogrel or shorten aspirin
DAPT: dual antiplatelet therapy; PCI: percutaneous coronary intervention
De-escalation is defined as switching from prasugrel or ticagrelor to clopidogrel as a strategy to reduce long-term bleeding events without reduction in ischemic protection and has been tested in several clinical trials (Table 2).5 This approach is based on the concept that, while antithrombotic benefit of P2Y12 inhibitors is greater during the early phase after ACS/PCI, the increase in bleeding risk associated with more potent P2Y12 inhibitors seems to accrue mainly in the chronic phase. A de-escalation strategy was first tested in the TOPIC trial, which showed that de-escalation at 30 days post-ACS was associated with a 52% reduction in a composite of bleeding and ischemic events, mainly driven by a reduction in major bleeding events, as compared with prolonged treatment with potent P2Y12 inhibitors.6 However, registry data have shown that unguided de-escalation was associated with increased risk of ischemic events.7 Therefore, due to conflicting results, a routine de-escalation approach may not be the optimal strategy, but de-escalation should be reserved for patients at higher bleeding risk. Although this latter approach seems intuitive, the only de-escalation trial specifically designed for high bleeding risk patients was the POPULAR AGE trial.8 This trial was performed in elderly (≥70 years old) patients with non-ST elevation-acute coronary syndrome (NSTE-ACS) and showed that clopidogrel was associated with a significant 6% absolute reduction in bleeding events compared to standard treatment with ticagrelor or prasugrel and was non-inferior for ischemic events.
Table 2: Clinical Trials on De-Escalation of DAPT Therapy
| Study | Design | N of patients | Treatment arms | Primary endpoint | Main findings |
| TOPIC6 | Open-label, single-center, randomized trial, 30 days post-ACS | 645 | Switched DAPT (prasugrel or ticagrelor to clopidogrel) vs. continued DAPT with prasugrel or ticagrelor) | Composite of cardiovascular death, urgent revascularization, stroke, and bleeding as defined by BARC ≥2 at 1 year post ACS | Primary endpoint: 13.4% in the switched DAPT group vs. 26.3% in the unchanged DAPT (HR 0.48; 95% CI 0.48 (0.34-0.68); p<0.01) |
| POPULAR AGE8 | Open-label, multi-center, randomized controlled trial in elderly patients (>70 years) with NSTE-ACS | 1002 | Ticagrelor or prasugrel vs. clopidogrel in patients | Composite of all-cause death, myocardial infarction, stroke, PLATO major and minor bleeding | Primary endpoint: clopidogrel 27.7% vs. ticagrelor/prasugrel 32.1%; HR 0.82; 95% CI 0.66 to 1.03; p=0.030 for non-inferiority PLATO major and minor bleeding: clopidogrel 18% vs. ticagrelor/prasugrel 24%; HR 0.71; 95% CI 0.54 to 0.94; p=0.018 for superiority |
| TROPICAL ACS10 | Multicenter, randomized clinical trial in biomarker-positive ACS patients after successful PCI | 2610 | PFT-Guided de-escalation to clopidogrel vs. continued prasugrel | Composite of cardiovascular death, myocardial infarction, stroke, and BARC bleeding ≥ grade 2 | Primary endpoint: 7% in the guided de-escalation group vs. 9% in the control group; HR 0.81; 95% CI 0.62-1.06; (p =0.0004 for non-inferiority; p=0.12 for superiority) |
| POPULAR GENETICS14 | Randomized, open-label, assessor-blinded trial in STEMI patients treated with primary PCI | 2488 | Genotype-guided therapy (ticagrelor or prasugrel in LoF alleles carriers and clopidogrel in noncarriers) vs. standard treatment with ticagrelor or prasugrel | Composite of death from any cause, myocardial infarction, definite stent thrombosis, stroke, or PLATO major bleeding | Primary endpoint: genotype-guided group 5.1% vs. standard treatment group 5.9%; HR 0.87; 95% CI 0.62-1.21; p<0.001 for noninferiority; p=0.40 for superiority) PLATO major and minor bleeding: genotype-guided group 9.8% vs. standard treatment group 12.5%; HR 0.78; 95% CI 0.61-0.98; p=0.04 for superiority |
Several studies have shown that potent P2Y12 inhibitors have enhanced ischemic benefit compared with clopidogrel particularly in patients with high platelet reactivity (HPR) or CYP2C19 loss-of-function (LoF) alleles. This has set the basis for using platelet function testing (PFT) and genetic testing in patients undergoing PCI to improve outcomes by tailoring antiplatelet treatment to the individual patient. Guided de-escalation with PFT or genetic testing has therefore become an emerging strategy that allows selecting clopidogrel poor responders, who can benefit the most from potent P2Y12 inhibition. The selective use of prasugrel or ticagrelor among clopidogrel poor responders has the potential to reduce bleeding complications related to the broad use of potent P2Y12 inhibitors while avoiding the increased ischemic risk associated with unselected use of clopidogrel. Guided de-escalation has been tested in several clinical trials.9
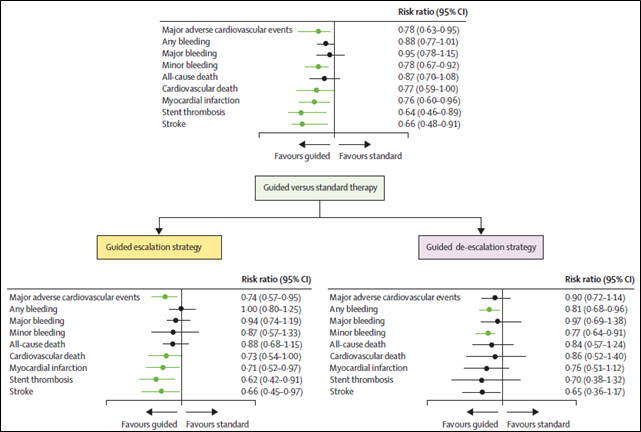
The TROPICAL-ACS trial evaluated a PFT-guided de-escalation strategy in patients with ACS undergoing PCI.10 The trial showed that PFT-guided de-escalation was non-inferior to unchanged prasugrel treatment on a composite end point including ischemic and bleeding events (7% in the guided de-escalation group vs. 9% in the control group; p non-inferiority=0.0004). BARC ≥2 bleeding was also numerically lower in the de-escalation group, without an increase in ischemic events. However, although PFT guided de-escalation has the advantage of defining the platelet phenotype that is more related to thrombosis (i.e., HPR), it also deals with inherent limitations, such as requiring patients to be on treatment to define responsiveness, variability in assays and results, and need for patients with HPR after de-escalation to escalate again to more potent P2Y12 inhibitors, which could represent a challenge in real-world clinical practice.11 Genetic testing for CYP2C19 LoF alleles can overcome these limitations given that the genetic profile of an individual remains unchanged and does not require a patient to be on treatment. Importantly, the commercial availability of rapid bedside genotyping assays has now made their implementation feasible in real-world practice.12,13 The use of genotype-guided de-escalation is supported by the results of the POPular Genetics trial, showing that in patients with ST-segment elevation myocardial infarction undergoing primary PCI, guided de-escalation was noninferior to a standard approach with respect to net adverse events (5.1% vs. 5.9; p<0.001 for noninferiority), with a significant reduction in major or minor bleeding events (9.8% vs. 12.5%; p=0.04).14 A meta-analysis by Pereira et al. compared the clinical effects of potent P2Y12 inhibitors (ticagrelor or prasugrel) versus clopidogrel taking into consideration the presence or absence of LoF alleles. Pooling together 15,949 patients, mostly ACS, from seven randomized controlled trials, the authors showed that the use of potent P2Y12 inhibitors was beneficial only in patients with LoF alleles, whereas it did not provide any additional benefit in patients without LoF alleles, supporting the potential benefit of genotype-guided de-escalation.15 A recent comprehensive meta-analysis pooling data from more than 20,000 patients showed that a strategy of guided selection of antiplatelet therapy significantly reduced the risk of major adverse cardiovascular events compared with standard antiplatelet therapy.16 In particular, the study showed that guided de-escalation of DAPT was superior in reducing bleeding events without increase in ischemic events (Figure 1), supporting the role of a precision-medicine approach in selecting antiplatelet therapy.
Figure 1: Guided (Platelet Function/Genetic Testing) versus Standard Antiplatelet Therapy in Patients Undergoing PCI: A Systematic Review and Meta-analysis
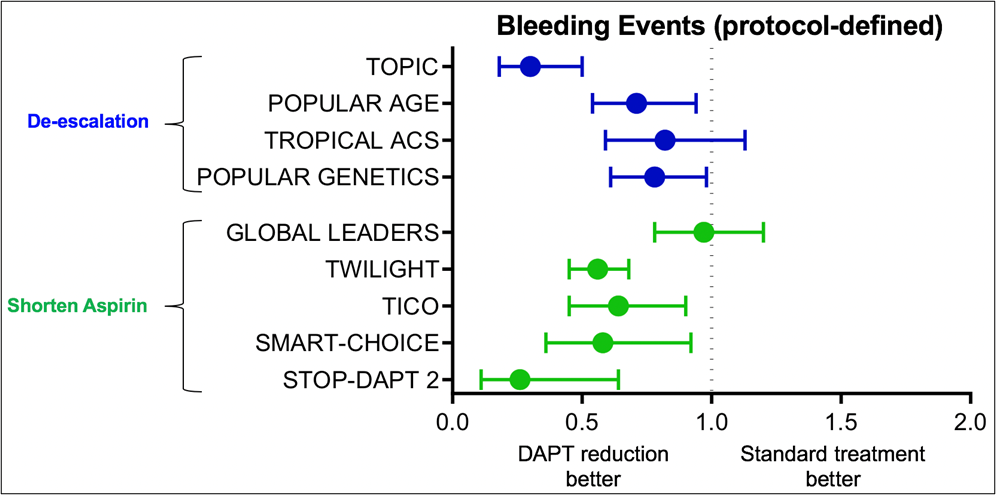
De-escalation of P2Y12 inhibitor therapy has recently been endorsed by guidelines as an alternative option in patients unsuitable for potent platelet inhibition.17 However, de-escalation is only one of the available strategies for bleeding reduction following PCI, and selecting the best option represents a clinical conundrum. When compared to shortening of aspirin duration, an emerging strategy tested in several recent clinical trials, both approaches show overall a reduction in bleeding without an increase in ischemic events (Figure 2). Therefore, the choice of the strategy should be based on the specific characteristics of the individual patient, including bleeding and thrombotic risk, affordability of potent P2Y12 inhibitors, and presence of clinical factors, including genotype, favoring poor response to clopidogrel. In addition, despite the report that in the presence of potent P2Y12 receptor blockade aspirin may provide little additional platelet inhibition, setting the basis for aspirin-free strategies,18 aspirin is known to have an important synergistic antithrombotic effect when used in combination with P2Y12 inhibitors. De-escalation, by preserving the synergistic inhibition of multiple pathways, might indeed be beneficial over other strategies in terms of ischemic protection.19
Figure 2: Comparison Between Bleeding Reduction Strategies in ACS/PCI
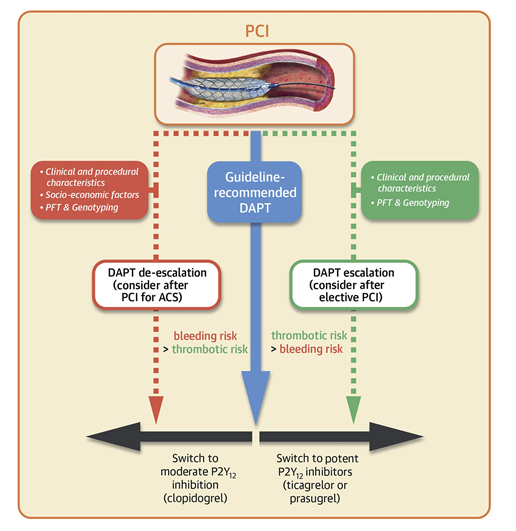
In conclusion, although de-escalation of P2Y12 inhibitors commonly occurs in clinical practice, routine de-escalation should not be recommended. This strategy should be considered in selected patients to reduce bleeding risk and based on the available evidence; an approached based PFT- or genotype-guided de-escalation should be preferred if possible (Figure 3). Because of the above-mentioned limitations of PFT and because genotype is only one of the factors involved in clopidogrel response variability, integrating genetic information with clinical and procedural variables have been shown to have greater accuracy than the individual components alone, and is likely to represent the most successful strategy for personalization of antiplatelet therapy in patients undergoing PCI.20 Due to high thrombotic risk, early (<30 days) de-escalation should be avoided, especially if unguided, and clinicians should be aware of the correct switching approach to avoid the risk of drug-drug interactions, which could potentially expose the patient to enhanced thrombotic risk.5
Figure 3: Dual Antiplatelet Therapy Strategies After PCI
References
- Franchi F, Angiolillo DJ. Novel antiplatelet agents in acute coronary syndrome. Nat Rev Cardiol 2015;12:30-47.
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045-57.
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001-15.
- Capodanno D, Morice MC, Angiolillo DJ, et al. Trial design principles for patients at high bleeding risk undergoing PCI: JACC Scientific Expert Panel. J Am Coll Cardiol 2020;76:1468-83.
- Angiolillo DJ, Rollini F, Storey RF, et al. International expert consensus on switching platelet P2Y12 receptor-inhibiting therapies. Circulation 2017;136:1955-75.
- Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J 2017;38:3070-78.
- De Luca L, D'Ascenzo F, Musumeci G, et al. Incidence and outcome of switching of oral platelet P2Y12 receptor inhibitors in patients with acute coronary syndromes undergoing percutaneous coronary intervention: the SCOPE registry. EuroIntervention 2017;13:459-66.
- Gimbel M, Qaderdan K, Willemsen L, et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomized, open-label, non-inferiority trial. Lancet 2020;395:1374-81.
- Galli M, Benenati S, Capodanno D, et al. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Lancet 2021;397:1470-83.
- Sibbing D, Aradi D, Jacobshagen C, et al. A randomized trial on platelet function-guided de-escalation of antiplatelet treatment in ACS patients undergoing PCI. Rationale and design of the Testing Responsiveness to Platelet Inhibition on Chronic Antiplatelet Treatment for Acute Coronary Syndromes (TROPICAL-ACS) trial. Thromb Haemost 2017;117:188-95.
- Sibbing D, Aradi D, Alexopoulos D, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y12 receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv 2019;12:1521-37.
- Franchi F, Rollini F. Genotype-guided antiplatelet therapy in patients with coronary artery disease. JACC Cardiovasc Interv 2021;14:751-53.
- Franchi F, Rollini F, Rivas J, et al. Prasugrel versus ticagrelor in patients with CYP2C19 loss-of-function genotypes: results of a randomized pharmacodynamic study in a feasibility investigation of rapid genetic testing. JACC Basic Transl Sci 2020;5:419-28.
- Claassens DMF, Vos GJA, Bergmeijer TO, et al. A genotype-guided strategy for oral P2Y12 inhibitors in primary PCI. N Engl J Med 2019;381:1621-31.
- Pereira NL, Rihal C, Lennon R, et al. Effect of CYP2C19 genotype on ischemic outcomes during oral P2Y12 inhibitor therapy: a meta-analysis. JACC Cardiovasc Interv 2021;14:739-50.
- Galli M, Benenati S, Capodanno D, et al. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Lancet 2021;397:1470-83.
- Collet JP, Thiele H, Barbato E, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289-1367.
- Capodanno D, Mehran R, Valgimigli M, et al. Aspirin-free strategies in cardiovascular disease and cardioembolic stroke prevention. Nat Rev Cardiol 2018;15:480-96.
- Franchi F, Rollini F, Faz G, et al. Pharmacodynamic effects of vorapaxar in prior myocardial infarction patients treated with potent oral P2Y12 receptor inhibitors with and without aspirin: results of the VORA-PRATIC study. J Am Heart Assoc 2020;9:e015865.
- Angiolillo DJ, Capodanno D, Danchin N, et al. Derivation, validation, and prognostic utility of a prediction rule for nonresponse to clopidogrel: The ABCD-GENE score. JACC Cardiovasc Interv 2020;13:606-17.
Clinical Topics: Acute Coronary Syndromes, Invasive Cardiovascular Angiography and Intervention, Stable Ischemic Heart Disease, Vascular Medicine, Interventions and ACS, Interventions and Vascular Medicine, Chronic Angina
Keywords: Acute Coronary Syndrome, Platelet Aggregation Inhibitors, Prasugrel Hydrochloride, Clopidogrel, Ticagrelor, Purinergic P2Y Receptor Antagonists, Fibrinolytic Agents, Cytochrome P-450 CYP2C19, Percutaneous Coronary Intervention, Aspirin, ST Elevation Myocardial Infarction, Genotype, Precision Medicine, Genetic Profile, Alleles, Control Groups, Thrombosis, Registries, Phenotype, Genetic Testing, Reference Standards, Hemorrhage, Blood Platelets, Drug Interactions, Pharmaceutical Preparations
< Back to Listings