Modulation of Cardiac Arrhythmogenesis by Epicardial Adipose Tissue
Quick Takes
- Epicardial adipose tissue accumulation is closely associated with atrial and ventricular arrhythmias and with electrocardiographic signs associated with arrhythmogenesis.
- Epicardial adipose tissue infiltrations in the myocardium separate cardiac bundles and create anatomical obstacles delaying activation. In addition, the disparate distribution of epicardial adipose tissue over the myocardium leads to heterogeneity of conduction. The resulting heterogeneous conduction slowing facilitates re-entrant arrhythmias.
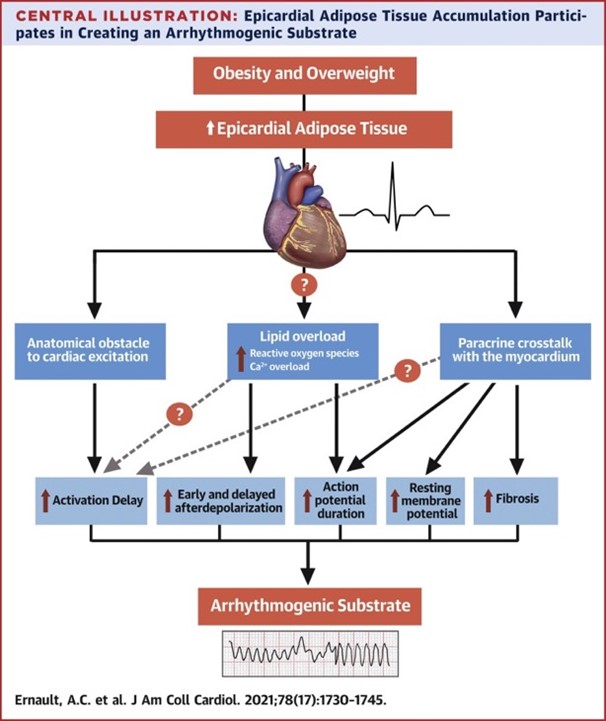
Obesity and overweight are a growing health problem leading to 2.8-4 million deaths every year, the majority relating to cardiovascular disease.1 Strong evidence shows that obesity plays an important role in arrhythmogenesis. Overweight has been associated with the amount of epicardial adipose tissue (EAT), of which the presence and volume is in turn related to atrial as well as ventricular arrhythmias.2 Although a growing body of published data documents the arrhythmogenicity of EAT, the underlying electrophysiological mechanisms are unknown. Based on reported studies, we identified important mechanisms by which EAT modulates cardiac arrhythmogenesis.
1. EAT and Lipotoxicity
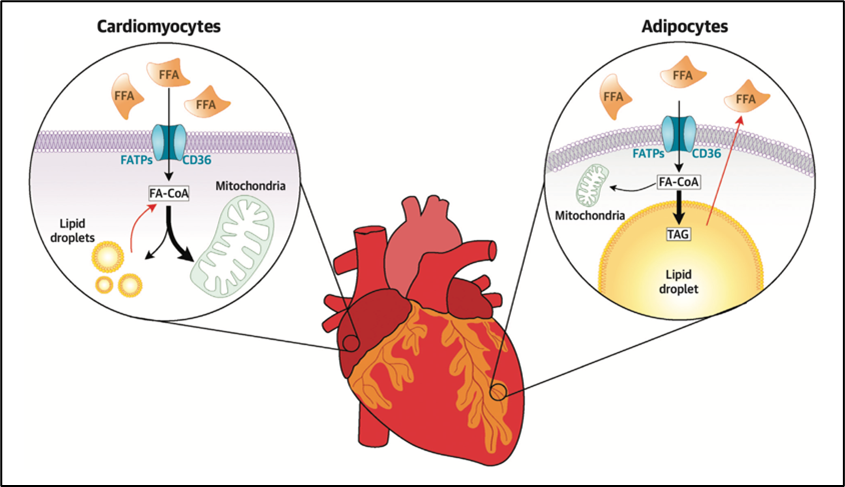
EAT refers to the visceral adipose tissue located between the myocardium and the epicardium, enclosed in the pericardial sac. Given the proximity between EAT and cardiomyocytes, the release of free fatty acids (FFAs) from adipocytes in EAT and into the plasma may act as a local and rapidly mobilizable cardiac energy supply (Figure 1).
Figure 1
In case of excess intracellular FFAs, the latter can be stored in myocardial cytosolic lipid droplets (LDs).3 Defects in LD formation or exceeding the LD capacity may be linked to an increased risk of arrhythmias.4 Indeed, although the heart preferentially uses lipids as metabolic substrate, lipid overload is toxic, leading to calcium dysregulation, and increased reactive oxygen species (ROS) production in the cardiomyocyte. Both calcium dysregulations and ROS increase the risk of arrhythmias based on the following mechanisms: (1) cytosolic calcium overload and spontaneous release of calcium could lead to delayed afterdepolarizations; (2) ROS can induce early afterdepolarizations and alter electrical coupling between cardiomyocytes, resulting in conduction slowing. Early and delayed afterdepolarizations facilitate triggered activity, whereas conduction slowing facilitates re-entry.
2. Structural Crosstalk Between EAT and Myocardium
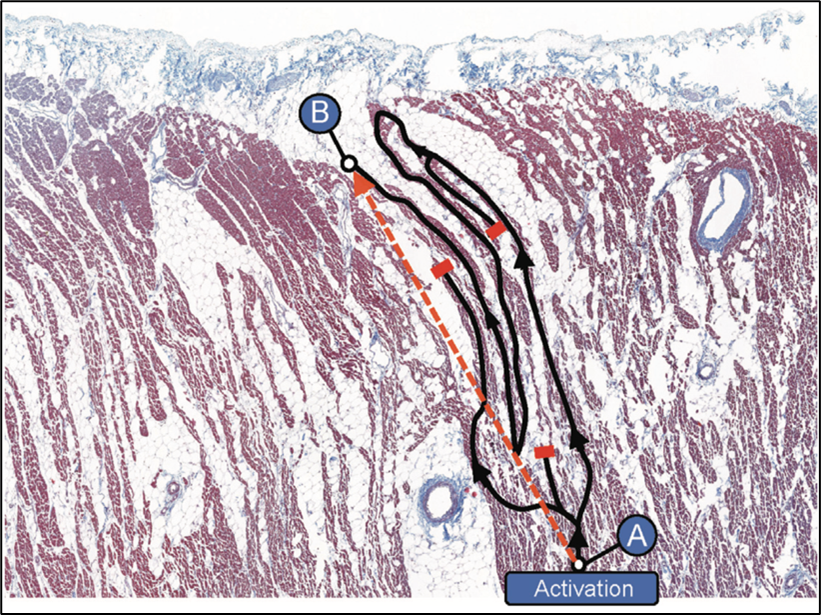
EAT forms an anatomical obstacle to cardiac excitation
Adipocyte infiltration into the myocardium separates cardiomyocytes from each other and creates an anatomical barrier, especially when fibrosis is induced (fibro-fatty infiltrations), forcing the electrical impulse to follow a "zigzag" path (Figure 2) similar to the observations in infarct scar tissue.2 In patients with coronary artery disease, fibro-fatty infiltrations have been associated with conduction delay and electrogram fractionation, increased fibrosis, and lateralization of connexin40, facilitating re-entry.5
Figure 2
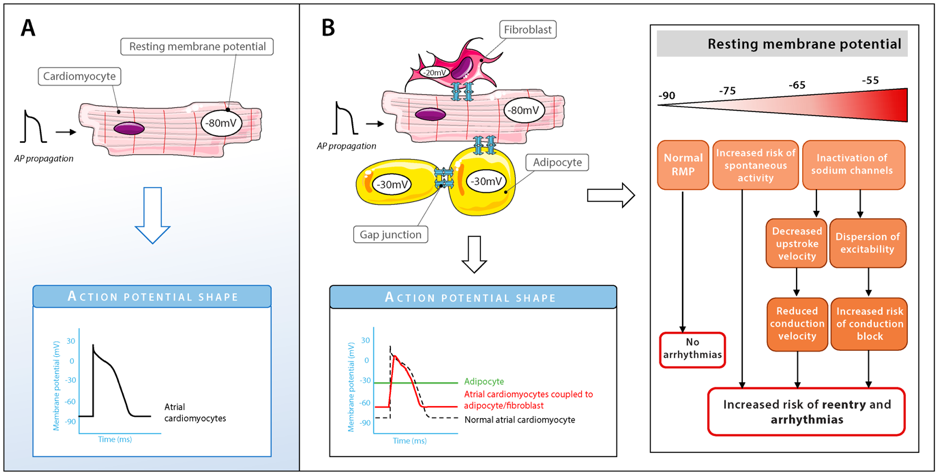
Electrical coupling between EAT cells and cardiomyocytes can facilitate arrhythmias
Adipocytes express gap junctional channel proteins such as Cx43, which are also highly expressed in cardiomyocytes. Whether adipocytes in EAT can electrically couple to cardiomyocytes is unknown. If so, electrical coupling between cardiomyocytes and adipocytes in EAT would result in respectively a depolarization and hyperpolarization of their resting membrane. Depending on the level of cardiac depolarization, its electrophysiological effect can range from increased excitability to (extreme) slowing of conduction or inexcitability (Figure 3). So far, no direct proof of cardiomyocyte-adipocyte coupling has been shown. Looking at studies investigating electrical coupling between cardiomyocyte and (myo)fibroblasts, which have similar membrane potentials as adipocytes, we could expect changes in action potential (AP) shapes (e.g., lower AP plateau levels and shortening of AP duration) and abnormal automaticity. However, further research is needed to understand whether intercellular coupling between EAT cells and cardiomyocytes can influence arrhythmogenicity.
Figure 3
3. Paracrine Crosstalk Between EAT and Myocardium
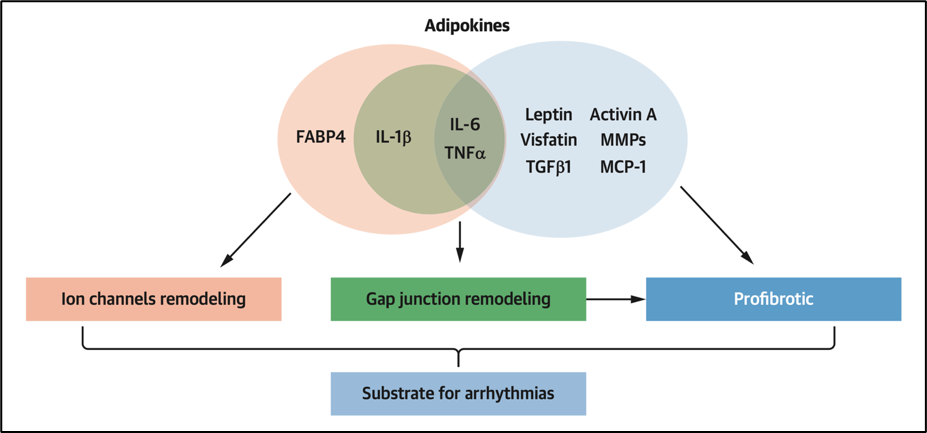
Cells communicate with each other through signaling molecules, including a variety of soluble factors (growth factors, cytokines, and bioactive lipids), some transported by extracellular vesicles (EVs) that carry various cargo (proteins, lipids, and signaling molecules). Indeed, adipose tissue is recognized as an endocrine organ secreting a large number of cytokines, growth factors, and hormones, known as "adipokines." Studies that have focused on the paracrine effects of EAT secretome on cardiac tissue are methodologically diverse leading to variable results.
- Several adipokines such as Activin-A have been shown to be pro-fibrotic7 and either involved in fibroblast proliferation, collagen synthesis, and/or myofibroblast activation in vitro.2 Fibrosis plays an important role as a potential substrate for arrhythmias by altering myocardial architecture leading to impaired conduction in a similar way as described above for the fibro-fatty infiltration.
- Other adipokines show potentially proarrhythmic effects on in vitro cardiomyocytes through alteration of potassium and calcium currents and by altering gap junctions, therefore directly impacting cardiac electrophysiology and the morphology and duration of the action potential. Heterogeneous AP duration (APD) changes constitute an increased risk of re-entry. Alterations in calcium dynamics can also facilitate re-entry and can additionally induce triggered activity by inducing early and delayed afterdepolarization.
Figure 4
Conclusion
In summary, the key mechanisms of arrhythmogenicity of EAT are:
- Metabolic: lipid overload is toxic, leading to calcium dysregulation, and increased ROS production, resulting in early and delayed afterdepolarization, APD prolongation and/or conduction slowing
- Structural: fibro-fatty replacement creates an anatomical barrier leading to heterogeneous anisotropic conduction
- Paracrine: EAT secretome lead to changes in APD and potentially APD heterogeneity and induces fibrosis leading to impaired conduction
Changes in APD (heterogeneity) and conduction slowing facilitate re-entry. Alterations in calcium dynamics can, besides facilitating re-entry via APD prolongation, also facilitate triggered activity by induction of early and delayed afterdepolarization. EAT is a potential target for prevention of cardiac remodelling and arrhythmias.
Translational Perspective
Several studies have reported a positive association between EAT volume and the duration as well as fragmentation and dispersion of both the P-wave and QRS-complex on the electrocardiogram (ECG). These ECG features reflect atrial and ventricular conduction delay and anisotropic, heterogeneous impulse propagation, respectively. We provide several mechanisms of crosstalk between cardiomyocytes and adipocytes that explain the increased risk of cardiac arrhythmias in individuals with increased EAT.
References
- Afshin A, Forouzanfar MH, Reitsma MB, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 2017;377:13-27.
- Ernault AC, Meijborg VMF, Coronel R. Modulation of cardiac arrhythmogenesis by epicardial adipose tissue: JACC State-of-the-Art Review. J Am Coll Cardiol 2021;78:1730-45.
- Sobczak AIS, Blindauer CA, Stewart AJ. Changes in plasma free fatty acids associated with type-2 diabetes. Nutrients 2019;11:2022.
- Schulze PC, Drosatos K, Goldberg IJ. Lipid use and misuse by the heart. Circ Res 2016;118:1736-51.
- Nalliah CJ, Bell JR, Raaijmakers AJA, et al. Epicardial adipose tissue accumulation confers atrial conduction abnormality. J Am Coll Cardiol 2020;76:1197-1211.
- Vigmond EJ, Efimov IR, Rentschler SL, Coronel R, Boukens BJ. Fractionated electrograms with ST-segment elevation recorded from the human right ventricular outflow tract. HeartRhythm Case Rep 2017;3:546–50.
- Venteclef N, Guglielmi V, Balse E, et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J 2015;36:795-805.
Clinical Topics: Arrhythmias and Clinical EP, Dyslipidemia, Heart Failure and Cardiomyopathies, Atherosclerotic Disease (CAD/PAD), Implantable Devices, EP Basic Science, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Lipid Metabolism, Diabetes and Cardiometabolic Disease, Pericardial Disease
Keywords: Reactive Oxygen Species, Myocytes, Cardiac, Calcium, Connexin 43, Fatty Acids, Nonesterified, Coronary Artery Disease, Cardiovascular Diseases, Action Potentials, Cicatrix, Electrophysiologic Techniques, Cardiac, Intra-Abdominal Fat, Lipid Droplets, Membrane Potentials, Myofibroblasts, Overweight, Ventricular Remodeling, Pericardium, Adipose Tissue, Activins, Adipokines, Arrhythmias, Cardiac, Electrocardiography, Extracellular Vesicles, Gap Junctions, Cell Proliferation, Ion Channels, Adipocytes, Cytokines, Potassium, Collagen, Infarction, Obesity
< Back to Listings