Simultaneous Versus Sequential Initiation of HFrEF Therapies
Quick Takes
- There is robust evidence behind the life-saving pharmacotherapies of chronic HFrEF, yet optimal utilization of such medications is lacking both in the United States and around the world.
- Historically, the mainstays of HFrEF therapy were started individually and titrated slowly; however, new evidence suggests that simultaneous initiation and rapid titration of comprehensive disease-modifying medical treatment – namely ARNI, SGLT2i, BB, and MRA – is safe, effective, and derives the maximum benefit for the patient.
- From an individual perspective, treatment pathways like the rapid sequence initiation of CDMMT can help HFrEF patients quickly and safely start on medications shown to improve cardiovascular outcomes, and from a societal perspective, such pathways can help fill the treatment gap and standardize universal HFrEF care.
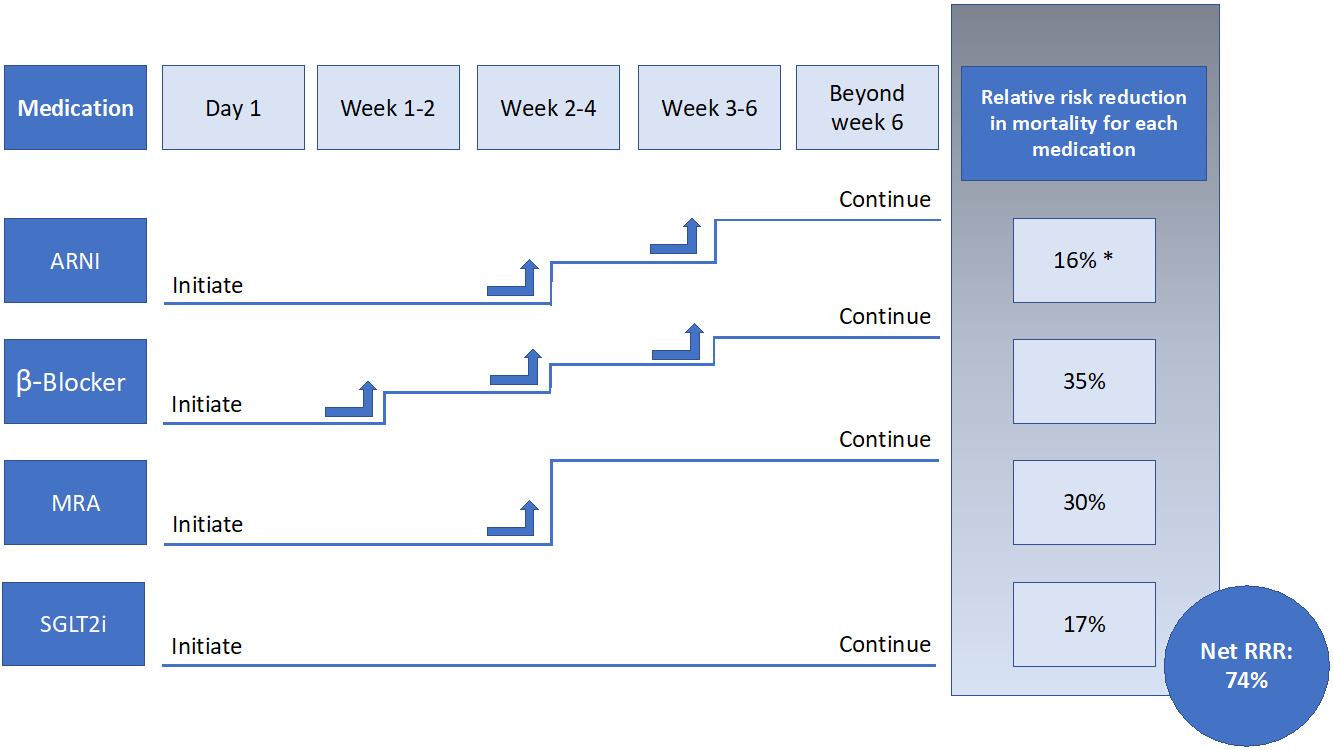
The evidence for pharmacotherapy improving cardiovascular outcomes in heart failure (HF) with reduced ejection fraction (HFrEF) dates back over 30 years, since the first trials of angiotensin-converting enzyme inhibitors (ACE-I).1 Subsequently, the use of guideline-directed medical therapy (GDMT) – comprised of an ACE-I or angiotensin receptor blocker (ARB), beta blocker (BB), and mineralocorticoid receptor antagonist (MRA) – became standard of care, with each medication providing additional survival benefit.2,3 More recently, comprehensive disease modifying medical therapy (CDMMT) – namely angiotensin receptor-neprilysin inhibitors (ARNI), sodium-glucose cotransporter 2 inhibitors (SGLT2i), BBs, and MRAs – have been established as the mainstays of HFrEF care, with estimates suggesting that compared to limited conventional therapy with an ACEI/ARB + BB, use of CDMMT could further reduce cardiovascular mortality in HFrEF by 50%.2-4
Despite these incredible advances in HFrEF treatment, utilization rates of both GDMT and CDMMT is dismal with large observational studies suggesting that pharmacotherapies are not being used at their optimal dosing, or worse, not being prescribed at all.5-7 Current estimates suggest that ACE-I/ARB usage in HFrEF is about 60-80%; ARNI usage is just above 10%; BB usage is about 60-80%; and MRA usage is about 30-60%. Worse still, target dosing is as low as 10-20% for ACEI/ARB, 10% for ARNI, 10-20% for BB, and 60-80% for MRA. Although SGLT2i are now formally recommended for HFrEF by both the American College of Cardiology (ACC) and the European Society of Cardiology (ESC), data surrounding current prescription patterns for HFrEF is not readily available.2,3
Underutilization matters. Adherence with HFrEF treatment reduces death due to HF, as well as cardiovascular death or hospitalization due to HF; in contrast, both nonadherence and underdosing have been associated with increased rates of HF decompensation and worse survival.8-10 If these medications are known to prolong life and improve quality of life, why are they not being prescribed? One possible concern for providers and patients alike is polypharmacy. However, evidence suggests that the individual components of CDMMT are safe to use concomitantly. In the PARADIGM-HF trial comparing ARNI to standard of care, over 90% of patients in the experimental arm were on BBs and over 50% were on MRAs; fewer patients in the experimental arm stopped their medication due to an adverse event, compared to the control arm of enalapril, though there was a run in phase to the trial.11 In DAPA-HF, 70-95% of patients were on an ACEI/ARB/ARNI, BB, or MRA, yet the adverse event rates were comparable between the experimental arm and the control arm.12 Furthermore, both ARNIs and SGLT2is, as well as the old mainstays of GDMT, have proved to be safe when used simultaneously, not only for outpatients, but also during hospitalization for HFrEF.13-16 Thus the current evidence suggests that a multi-drug treatment regimen is both safe and well-tolerated.
Other issues may include cost, particularly with the more novel SGLT2is and ARNIs. Insurance coverage remains a barrier for these medications in the United States (US), where prior authorization forms and high copays hinder medication access at both the physician and the patient level.2 However, it should be noted that these medications are considered high value by recent cost-effectiveness analyses, despite their present increased price.13 Further concerns exist for adverse effects and medication intolerance; as a result, HFrEF medications were historically started sequentially, with one medication initiated and titrated to maximal dose before another medication was added.17 This strategy may allow for closer monitoring of the patient's clinical status and tolerance to each individual therapy, but it delays life-prolonging treatment. This slow-and-steady approach also leads to clinical inertia, titration fatigue, and a reluctance to increase medications to target dosing. Whether due to a loss of urgency or a loss of motivation, clinicians may develop a false sense of security in the "clinically stable" patient, who as a result of their clinical status, does not receive further medication initiation or titration.18
An alternative approach is thus needed, and one strategy to improve utilization of CDMMT is the simultaneous/rapid sequence initiation followed by titration of HFrEF therapies, as tolerated.2,3,13,17,19 Because the reduction in cardiovascular endpoints with CDMMT can occur as quickly as 2-6 weeks after the start date, simultaneous/rapid sequence initiation of all medications – as opposed to the slower serial stepwise approach – is preferred.13 Such a pathway to initiate CDMMT has been described in prior publications and guidelines.2,3,13,17 All four medications – ARNIs, SGLT2is, BBs, and MRAs – can be started simultaneously or in rapid sequence, including in an inpatient setting, so long as the patient is hemodynamically stable. These medications should be initiated at the lowest dose, and up-titrated every 1-2 weeks as tolerated (Figure 1). Monitoring of blood pressure, electrolytes, and renal function should be done throughout up-titration. Ideally within 6-8 weeks, a HFrEF patient would be receiving target doses of CDMMT as tolerated.
Figure 1
While initiating medications in a prolonged sequence is tolerated based on clinical trials and observational data, and some would advocate for trials evaluating initiation strategies, there is no evidence this approach is any safer or more beneficial than a simultaneous/rapid sequence initiation strategy.2,3,17 Therefore, in order to improve utilization for all patients with HFrEF and rapidly improve clinical outcomes, CDMMT should be started simultaneously/in rapid sequence in eligible patients and titrated as tolerated without a significant increase in adverse safety events. Such a strategy allows for maximal therapeutic benefit in a shorter period, with less confusion surrounding medication switching, initiation, and dose intensification.
Estimates suggest that optimal use of GDMT in the US could potentially save 98,000 lives annually; use of ARNIs and SGLT2is could save 28,000 and 34,000 lives, respectively.13,20,21 It is thus of paramount importance that treatment for HFrEF be started quickly and up-titrated rapidly.
References
- Burnett H, Earley A, Voors AA, et al. Thirty years of evidence on the efficacy of drug treatments for chronic heart failure with reduced ejection fraction: a network meta-analysis. Circ Heart Fail 2017;10:e0003529.
- Maddox TM, Januzzi JL, Allen LA, et al. 2021 update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction. J Am Coll Cardiol 2021;77:772-810.
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599-3726.
- Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet 2020;396:121-28.
- Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF Registry. J Am Coll Cardiol 2018;72:351-66.
- Maddox TM, Song Y, Allen J, et al. Trends in U.S. ambulatory cardiovascular care 2013 to 2017: JACC Review Topic of the Week. J Am Coll Cardiol 2020;75:93-112.
- Savarese G, Bodegard J, Norhammar A, et al. Heart failure drug titration, discontinuation, mortality and heart failure hospitalization risk: a multinational observational study (US, UK and Sweden). Eur J Heart Fail 2021;23:1499-1511.
- Komajda M, Schöpe J, Wagenpfeil S, et al. Physicians' guideline adherence is associated with long‐term heart failure mortality in outpatients with heart failure with reduced ejection fraction: the QUALIFY international registry. Eur J Heart Fail 2019;21:921-29.
- Ouwerkerk W, Voors AA, Anker SD, et al. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: a prospective European study. Eur Heart J 2017;38:1883-90.
- Butler J, Yang M, Manzi MA, et al. Clinical course of patients with worsening heart failure with reduced ejection fraction. J Am Coll Cardiol 2019;73:935-44.
- McMurray JJV, Packer M, Desai AS, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993-1004.
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995-2008.
- Brownell NK, Ziaeian B, Fonarow GC. The gap to fill: rationale for rapid initiation and optimal titration of comprehensive disease-modifying medical therapy for heart failure with reduced ejection fraction. Card Fail Rev 2021;7:e18.
- Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin–neprilysin inhibition in acute decompensated heart failure. N Engl J Med 2019;380:539-48.
- Morrow DA, Velazquez EJ, DeVore AD, et al. Clinical outcomes in patients with acute decompensated heart failure randomly assigned to sacubitril/valsartan or enalapril in the PIONEER-HF Trial. Circulation 2019;139:2285-88.
- Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117-28.
- Greene SJ, Butler J, Fonarow GC. Simultaneous or rapid sequence initiation of quadruple medical therapy for heart failure—optimizing therapy with the need for speed. JAMA Cardiol 2021;6:743-44.
- Fiuzat M, Ezekowitz J, Alemayehu W, et al. Assessment of limitations to optimization of guideline-directed medical therapy in heart failure from the GUIDE-IT trial: a secondary analysis of a randomized clinical trial. JAMA Cardiol 2020;5:757-64.
- Bhagat AA, Greene SJ, Vaduganathan M, Fonarow GC, Butler J. Initiation, continuation, switching, and withdrawal of heart failure medical therapies during hospitalization. JACC Heart Fail 2019;7:1-12.
- Fonarow GC, Hernandez AF, Solomon SD, Yancy CW. Potential mortality reduction with optimal implementation of angiotensin receptor neprilysin inhibitor therapy in heart failure. JAMA Cardiol 2016;1:714-17.
- Bassi NS, Ziaeian B, Yancy CW, Fonarow GC. Association of optimal implementation of sodium-glucose cotransporter 2 inhibitor therapy with outcome for patients with heart failure. JAMA Cardiol 2020;5:948-51.
Clinical Topics: Cardiovascular Care Team, Heart Failure and Cardiomyopathies, Acute Heart Failure, Heart Failure and Cardiac Biomarkers
Keywords: Angiotensin Receptor Antagonists, Mineralocorticoid Receptor Antagonists, Neprilysin, Sodium-Glucose Transporter 2 Inhibitors, Quality of Life, Stroke Volume, Heart Failure, Diabetes Mellitus, Type 2, Blood Pressure, Inpatients, Outpatients, Polypharmacy, Prior Authorization, Standard of Care, Risk, Angiotensin-Converting Enzyme Inhibitors, Hospitalization, Receptors, Angiotensin, Insurance Coverage, Contraindications, Health Services Accessibility, Kidney, Prescriptions, Electrolytes, Enalapril, Fatigue, Sodium, Pharmaceutical Preparations, Cost-Benefit Analysis
< Back to Listings