Cardiotoxicity and the Evolving Landscape of HER2-Targeted Breast Cancer Treatment
Quick Takes
- The landscape of cancer treatment is evolving with the introduction of novel therapeutics and strategies for tailoring or de-escalating treatment regimens. It is therefore critical that current clinical practices for the prevention, diagnosis, and treatment of cardiotoxicity also adapt to this evolving landscape of breast cancer care.
- Breast cancer survivors treated with cardiotoxic cancer therapies remain at risk for early- and late-cardiotoxicity. Ongoing efforts are needed to identify patients at high-risk for cardiotoxicity and to develop effective diagnostic and therapeutic strategies aimed at improving clinical outcomes in the growing population of breast cancer survivors.
Background
Advances in cancer diagnosis and treatment have led to a decline in cancer deaths and growth in the cancer survivor population which is expected to exceed 22 million over the next 10 years.1 As cancer survivors live for many years beyond treatment and are exposed to the risks of early and late treatment-related complications, there is a growing need to mitigate the adverse cardiovascular effects of cancer treatment (from here on referred to as cardiotoxicity). Cardiotoxicity of breast cancer therapies have been well described, particularly for anthracyclines, human epidermal growth factor receptor 2 (HER2) targeted therapies (e.g., antibodies, tyrosine kinase inhibitors, antibody-drug conjugates), and radiotherapy. In this brief review, we provide a focused update on the changing landscape of HER2-positive breast cancer treatment and progress in the diagnosis, prevention, and treatment of cardiotoxicity (Figure 1).
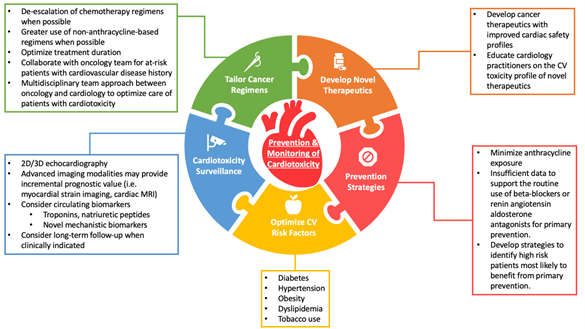
Figure 1: Current Approaches For Cardiotoxicity Prevention and Surveillance
Tailoring Breast Cancer Treatment
HER2-targeted therapies such as trastuzumab improve survival and reduce recurrence in patients with HER2-positive breast cancer but confer a risk of cardiotoxicity, most commonly manifested by a reduction in left ventricular ejection fraction (LVEF) with or without clinical symptoms of heart failure.2 While HER2-targeted therapy is typically delivered for 12 months in the early-stage setting, several randomized controlled trials have evaluated whether shorter durations of HER2-targeted treatment can achieve similar cancer control but with reduced toxicity. Although several trials (e.g. PHARE, SOLD, SHORT-HER, and HORG) failed to demonstrate that shorter durations of trastuzumab are non-inferior to the standard 12-month treatment,3-6 differing results from the PERSEPHONE study suggest that 6 months of adjuvant trastuzumab is non-inferior to 12 months, prompting considerable debate.7,8 Proposed explanations for this discrepancy include differences in study design and statistical methods, variability in the prespecified non-inferiority margin, and heterogeneity of study populations between studies. A meta-analysis by Chen et al. of more than 11,000 patients concluded that 1-year of trastuzumab conferred substantial disease-free and overall survival benefit compared to shorter treatments, but was associated with increased cardiotoxicity.9 While these data support the current 12-month duration of trastuzumab, they also suggest the potential for individualized treatment decisions for patients at high-risk for cardiotoxicity or those with early subclinical cardiotoxicity.
Recent efforts to tailor the treatment strategy for HER2-positive breast cancer include clinical trials in which therapy is escalated or de-escalated as needed based upon underlying risk of cancer recurrence or early treatment response to initial therapy. The APT trial by Tolaney et al. studied a treatment strategy of adjuvant paclitaxel and trastuzumab in 406 low-risk patients with stage I HER2-positive disease and reported a 3-year invasive disease free survival of 98.7%, with only 0.5% symptomatic heart failure and 2.2% asymptomatic decline of LVEF.10,11 Based upon results from this single-arm phase 2 study, paclitaxel and trastuzumab is currently a preferred treatment regimen for most patients with stage I disease that is both effective and safe. In a similar de-escalation study, the TRAIN-2 trial demonstrated similar treatment outcomes with or without anthracyclines in patients with stage II-III HER2-postive breast cancer receiving dual HER2-blockade.12 The CompassHER2 pCR study is investigating whether patients with stage II/IIIA HER2-positive breast cancer can safely receive less chemotherapy (e.g., omission of anthracycline chemotherapy) without compromising long-term survival. We anticipate that ongoing efforts to incorporate advanced molecular or radiologic techniques with traditional clinical risk predictors will help clinicians to further tailor treatment regimens and reduce adverse cardiovascular consequences of breast cancer treatment.
Novel HER2-Targeted Therapies
Novel HER2-targeted therapies have been developed for the treatment of HER2-positive breast cancer including oral tyrosine kinase inhibitors (i.e., neratinib) and antibody-drug conjugates (i.e., ado-trastuzumab emtansine [T-DM1] and trastuzumab deruxtecan [T-DXd]). The ExteNET trial showed that 1 year of extended adjuvant therapy with neratinib after completion of adjuvant trastuzumab-based therapy reduces the risk of invasive breast cancer recurrence, with no evidence of increased cardiotoxicity.13 Among patients with HER2-positive breast cancer and residual disease after completing neoadjuvant therapy, the KATHERINE trial showed that T-DM1 reduces risk of breast cancer recurrence or death compared to trastuzumab alone but without excess cardiotoxicity risk.14 Most recently, the efficacy and cardiac safety of T-DXd was reported in a phase 2 trial of 184 patients with metastatic HER2-positive breast cancer, in which only 3 (1.6%) developed left ventricular dysfunction.15 Overall these trials suggest that the risk of cardiotoxicity from newer HER2-targeted agents is low, although real-world clinical experience remains limited and warrants further investigation.
Cardiotoxicity Prevention and Management
Multiple prospective trials have evaluated the efficacy of beta-blockers, angiotensin-converting enzyme (ACE) inhibitors, or angiotensin receptor blockers (ARBs) for the primary prevention of cardiotoxicity. Interim results from the SAFE trial suggest the potential benefit of cardioprotective therapies (i.e., beta-blockers or ACE-inhibitors) for patients with breast cancer receiving anthracycline-based chemotherapy, consistent with findings from several prior studies (i.e. CECCY, PRADA, MANTICORE).16-19 A study by Guglin et al. has further shown that lisinopril and carvedilol are effective for preventing LVEF decline among a subset of patients treated with sequential anthracyclines and trastuzumab.20 These data, however, remain insufficient to support the routine use of cardioprotective medications for primary prevention, and ongoing studies are needed to identify patients who would be most likely to benefit from this approach.
Given the substantial clinical benefit of HER2-targeted therapy and the concern that early treatment interruption may portend to poorer cancer outcomes, two clinical trials have evaluated the safety of continuing HER2-targed therapy for patients with cardiotoxicity. In the SAFETY trial by Leong et al., 18 (90%) of 20 patients with mild cardiotoxicity who were treated with beta-blockers and ACE-inhibitors successfully completed all planned trastuzumab doses without worsening LVEF or development of heart failure.21 The SAFE-HEaRT study reported similar cardiac safety data, with 27 (90%) of 30 patients successfully completing all planned HER2-targted treatment without developing a significant decline of LVEF or clinical heart failure.22 Both trials are limited by small sample size but offer important preliminary data on the feasibility of continuing trastuzumab in patients with mild cardiotoxicity.
Cardiotoxicity Surveillance
Current recommendations for cardiotoxicity surveillance during breast cancer therapy include a LVEF assessment at baseline and at regular intervals during treatment, although data to support this practice is limited. Our group conducted a case-control study of 53 patients who developed cardiotoxicity during HER2-positive breast cancer and 159 cardiotoxicity-free controls and showed that a reduction in LVEF <55% detected at any surveillance time-point during HER2-positive breast cancer treatment was associated with increased risk for heart failure.23 These findings suggest that early detection of a reduced LVEF during routine surveillance may allow for implementation of strategies to prevent heart failure.
There has been growing body of evidence to support the prognostic value of global longitudinal strain (GLS) to detect early subclinical cardiac dysfunction during cancer treatment. The SUCCOUR study was a randomized controlled trial to determine whether a GLS-guided strategy for early cardioprotective therapy prevents reductions in LVEF among patients receiving cardiotoxic chemotherapy compared with a standard LVEF-based approach.24 The SUCCOUR trial failed to show a difference in the primary outcome of change in LVEF from baseline to 1 year between the treatment groups. However, the cumulative incidence of cardiotoxicity was lower in the GLS-guided arm compared to the LVEF-guided arm (5.8% vs. 13.7%, p=0.02). Additional studies are needed to further elucidate whether a GLS-guided strategy translates to improvement in long-term oncologic or cardiovascular clinical outcomes.
Conclusion
Cancer therapies associated with cardiotoxicity risk including anthracyclines and HER2-targeted agents remain important cornerstones of curative and life-prolonging breast cancer treatment. Ongoing efforts to optimize and de-escalate cancer treatment will reduce patient exposure to unnecessary cancer treatments and their associated toxicities. It is important that cardio-oncology practices for the prevention, surveillance, and treatment of cardiotoxicity adapt to the changing landscape of breast cancer treatment, particularly with the introduction of new therapeutics or modification of existing regimens. Future studies are needed to determine whether advanced imaging techniques or novel predictive biomarkers to current practices can be used to more effectively tailor cardio-oncology interventions (i.e., cardioprotective therapy or cardiotoxicity surveillance) to the right patients.
References
- Cancer Facts & Figures 2021 (cancer.org). 2021. Available at: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html. Accessed 04/15/2022.
- Copeland-Halperin RS, Liu JE, Yu AF. Cardiotoxicity of HER2-targeted therapies. Curr Opin Cardiol 2019;34:451-58.
- Conte P, Frassoldati A, Bisagni G, et al. Nine weeks versus 1 year adjuvant trastuzumab in combination with chemotherapy: final results of the phase III randomized Short-HER studydouble dagger. Ann Oncol 2018;29:2328-33.
- Joensuu H, Fraser J, Wildiers H, et al. Effect of ddjuvant trastuzumab for a duration of 9 weeks vs 1 year with concomitant chemotherapy for early human epidermal growth factor receptor 2-positive breast cancer: the SOLD randomized clinical trial. JAMA Oncol 2018;4:1199-1206.
- Mavroudis D, Saloustros E, Malamous N, et al. Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: a multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann Oncol 2015;26:1333-40.
- Pivot X, Romieu G, Debled M, et al. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet 2019;393:2591-98.
- Ponde N, Gelber RD, Piccart M. PERSEPHONE: are we ready to de-escalate adjuvant trastuzumab for HER2-positive breast cancer? NPJ Breast Cancer 2019;5:1.
- Earl HM, Hiller L, Vallier AL, et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet 2019;393:2599-2612.
- Chen L, Zhou W, Hu X, Yi M, Ye C, Yao G. Short-duration versus 1-year adjuvant trastuzumab in early HER2 positive breast cancer: a meta-analysis of randomized controlled trials. Cancer Treat Rev 2019;75:12-19.
- Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 2015;372:134-41.
- Dang C, Guo H, Najita J, et al. Cardiac outcomes of patients receiving adjuvant weekly paclitaxel and trastuzumab for node-negative, ERBB2-positive breast cancer. JAMA Oncol 2016;2:29-36.
- van Ramshorst MS, van der Voort A, van Werkhoven ED, et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2018;19:1630-40.
- Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18:1688-1700.
- von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 2019;380:617-28.
- Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med 2020;382:610-21.
- Gulati G, Heck SL, Ree AH, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): a 2 x 2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Eur Heart J 2016;3:1671-80.
- Pituskin E, Mackey JR, Koshman S, et al. Multidisciplinary approach to novel therapies in cardio-oncology research (MANTICORE 101-Breast): a randomized trial for the prevention of trastuzumab-associated cardiotoxicity. J Clin Oncol 2017;35:870-77.
- Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR Jr, et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: the CECCY trial. J Am Coll Cardiol 2018;71:2281-90.
- Livi L, Barletta G, Martella F, et al. Cardioprotective strategy for patients with nonmetastatic breast cancer who are receiving an anthracycline-based chemotherapy: a randomized clinical trial. JAMA Oncol 2021;7:1544-49.
- Guglin M, Krischer J, Tamura R, et al. Randomized trial of lisinopril versus carvedilol to prevent trastuzumab cardiotoxicity in patients with breast cancer. J Am Coll Cardiol 2019;73:2859-68.
- Leong DP, Cosman T, Alhussein MM, et al. Safety of continuing trastuzumab despite mild cardiotoxicity: a phase I trial. JACC CardioOncol 2019;1:1-10.
- Lynce F, Barac A, Tan MT, et al. SAFE-HEaRt: rationale and design of a pilot study investigating cardiac safety of HER2 targeted therapy in patients with HER2-positive breast cancer and reduced left ventricular function. Oncologist 2017;22:518-25.
- Yu AF, Moskowitz CS, Chuy KL, et al. Cardiotoxicity surveillance and risk of heart failure during HER2 targeted therapy. JACC CardioOncol 2020;2:166-75.
- Thavendiranathan P, Negishi T, Somerset E, et al. Strain-guided management of potentially cardiotoxic cancer therapy. J Am Coll Cardiol 2021;77:392-401.
Clinical Topics: Cardio-Oncology, Cardiovascular Care Team, Heart Failure and Cardiomyopathies, Prevention, Novel Agents, Statins, Acute Heart Failure
Keywords: Stroke Volume, Ado-Trastuzumab Emtansine, Angiotensin Receptor Antagonists, Cancer Survivors, Carvedilol, Lisinopril, Cardiotoxicity, Prospective Studies, Breast Neoplasms, Case-Control Studies, Disease-Free Survival, Feasibility Studies, Neoadjuvant Therapy, Preliminary Data, Ventricular Function, Left, Angiotensin-Converting Enzyme Inhibitors, Trastuzumab, Anthracyclines, Ventricular Dysfunction, Left, Heart Failure, Protein Kinase Inhibitors, Primary Prevention, Angiotensins, Paclitaxel
< Back to Listings