Vascular Toxicities of Novel Cancer Therapies
Introduction
Numerous types of cancer treatments, ranging from immunotherapy, traditional chemotherapy (e.g., doxorubicin), and targeted therapy (e.g., tyrosine kinase inhibitors [TKIs]) are associated with vascular toxicities. As cardiovascular disease (CVD) remains the leading cause of morbidity and mortality in patients with and without cancer, there is an urgent need to better understand the mechanisms and manifestations of vascular side effects to improve the care of cancer patients. In this brief review, we will summarize the common vascular toxicities associated with cancer therapies and provide a broad overview on their management.
Systemic Hypertension
Systemic hypertension is a common vascular toxicity due to vascular endothelial growth factor (VEGF) signaling pathway inhibition and is also a clinical marker of therapy efficacy.1 VEGF binds to VEGF receptors (VEGFR), promoting angiogenesis.2,3 VEGF signaling pathway inhibitors (VSPi) take advantage of tumor cells' dependence on blood supply by inhibiting VEGF signaling through various mechanisms of action: VEGF antibodies (e.g., bevacizumab), VEGFR antibodies (e.g., ramucirumab), and VEGFR TKIs (e.g., sorafenib, sunitinib) (Table 1).4 VSPi-associated hypertension is an "on-target" effect, affecting up to 90% of patients with newer generation VSPi (e.g., lenvatinib, lucitanib, axitinib).5,6 Life-threatening hypertensive crises, such as posterior reversible encephalopathy, are rare.7 Patients with pre-existing hypertension, advanced age (>60 years), tobacco use, hyperlipidemia, and obesity are at highest risk for VSPi associated hypertension.8,9 Mechanisms have been reviewed previously4,10 and are summarized in Table 2 below.
Table 1: VEGF Signaling Pathway (VSP) Inhibitors
| Target | Name | Mechanisms | Cardiovascular Toxicities | Cancer(s) |
| VEGF-A | Bevacizumab (Avastin) | Monoclonal antibody (mAb); humanized anti-VEGF antibody | Hypertension Arterial and venous thromboembolism Reversible cardiomyopathy Congestive heart failure Cardiac hypertrophy Myocardial infarction Cerebral ischemia Bleeding Proteinuria |
Metastatic colorectal cancer Advanced non-squamous non-small cell lung cancer Metastatic renal cell carcinoma Recurrent glioblastoma Advanced cervical cancer |
| VEGFR2 | Ramucirumab | mAb; binds to VEGFR-2 and blocks binding of VEGFR ligands, such as VEGF-A, VEGF-C, and VEGF-D | Hypertension Reversible cardiomyopathy Arterial and venous thromboembolism Bleeding Peripheral edema Proteinuria |
Advanced gastric or GE junction adenocarcinoma Metastatic NSCLC Metastatic colorectal cancer Advanced hepatocellular carcinoma |
| VEGFR1 | Icrucumab | mAb; binds to VEGFR-1 and inhibits downstream signaling | Peripheral edema Anemia No cardiotoxicity or hypertension identified |
Under clinical investigation for advanced solid cancers (e.g., small cell lung cancer, colorectal cancer) |
| VEGF | Ziv-aflibercept | VEGF-trap; peptide-antibody fusion protein that binds to circulating VEGF members (VEGF-A, VEGF-B) and placental growth factor | Hypertension Arterial and venous thromboembolism Cardiomyopathy Bleeding Proteinuria |
Metastatic colorectal cancer |
| Multi-target Tyrosine Kinase Inhibitors (TKI) | Axitinib | VEGFR1, 2, 3; PDGFR; c-KIT | Hypertension Cardiomyopathy Arterial and venous thromboembolism Bleeding Proteinuria |
Advanced RCC, advanced neuroendocrine tumors of non-pancreatic origin |
| Cabozantinib | VEGFR1, 2, 3, RET, FLT3; c-MET, AXL | Hypertension Cardiomyopathy Arterial and venous thromboembolism Bleeding Proteinuria |
Advanced RCC Metastatic medullary thyroid cancer Hepatocellular cancer |
|
| Lenvatinib | VEGFR 1, 2, 3; FGFR 1-4, PDGFRA, RET | Hypertension Cardiomyopathy Arterial and venous thromboembolism Bleeding QT prolongation Proteinuria |
Iodine-131-refractory thyroid cancer | |
| Cediranib | VEGFR1 2, 3; c-KIT; PDGFR | Hypertension Arterial and venous thromboembolism QT prolongation Proteinuria |
Advanced RCC, GIST, soft tissue sarcoma, HCC, advanced non-small cell lung cancer, advanced colorectal cancer, mesothelioma, breast cancer, ovarian cancer , GBM | |
| Nintedanib | PDGFR, FGFR, VEGFR-1, 2, 3; FLT3 | Hypertension Arterial and venous thromboembolism Myocardial infarction Bleeding Proteinuria |
Locally advanced, metastatic, or locally recurring non-small cell lung cancer (in combination with docetaxel); interstitial lung disease | |
| Pazopanib | VEGFR 1, 2, 3, PDGFR, FGFR, c-KIT, Flt-3, RET | Hypertension Cardiomyopathy Thromboembolism QT prolongation Torsades de pointes Proteinuria |
Metastatic medullary thyroid cancer Advanced renal cell carcinoma Advanced soft tissue sarcomas |
|
| Sunitinib | VEGFR 1, 2, 3, PDGFR-A and -B, c-KIT, RET, CD114, CD135 | Hypertension Reversible cardiomyopathy Arterial and venous thromboembolism Cardiac ischemia QT prolongation Proteinuria |
Advanced RCC, Progressive well-differentiated pancreatic neuroendocrine tumors, GIST | |
| Sorafenib | RAF kinase, VEGFR 1, 2, 3, PDGFR-B, RET, c-KIT, FLT3 | Hypertension Reversible cardiomyopathy Cardiac ischemia Arterial and venous thromboembolism Bleeding QT prolongation Proteinuria |
Advanced RCC, advanced unresectable HCC, GIST, angiosarcoma, advanced thyroid carcinoma refractory to radioactive iodine treatment | |
| Apatinib | VEGFR2, c-KIT, c-SRC | Hypertension Bleeding Left ventricular dysfunction Proteinuria |
Metastatic gastric carcinoma, metastatic breast cancer; advanced HCC, refractory metastatic colorectal cancer | |
| Lucitanib | VEGFR1, 2, 3, PGFRA/B, FGFR1 and 2 | Hypertension QT prolongation Proteinuria |
Advanced solid tumors (in clinical trials) | |
| Regorafenib | VEGFR 1, 2,3; TIE-2, RET, PDGFB, basic FGF-1, c-KIT, RAF-1, BRAF | Hypertension Thrombosis Heart failure Bleeding Proteinuria |
Advanced HCC, Advanced GIST, metastatic colorectal cancer | |
| Vandetanib | VEGFR-2, 3, EGFR, PDGFR, RET | Hypertension Cardiomyopathy QT prolongation |
Advanced RCC, medullary thyroid cancer, NSCLC | |
| Vatalanib | VEGFR1, 2, 3; PDGFR, c-KIT | Hypertension Heart failure Venous thromboembolism |
Advanced solid tumors | |
| Surufatinib |
VEGFR1, 2, 3; FGFR1, CSF1R | Hypertension Proteinuria Hypertriglyceridemia |
Advanced solid tumors; advanced medullary thyroid cancer | |
| Famitinib | VEGFR2 and 3, PDGFR, c-KIT, FGFR | Hypertension | Advanced genitourinary and gynecologic cancers | |
| Ponatinib | BCR-ABL, FGFR, VEGFR1, 2, 3, PDGFR, c-KIT, RET, FLT3 | Hypertension Arterial and venous thromboembolism Cardiac ischemia Atrial fibrillation Proteinuria |
Resistant Philadelphia chromosome-positive chronic myelogenous leukemia and acute lymphocytic leukemia |
Prior to initiation of VSPi, baseline blood pressures should be optimized (goal <130/80 mmHg) with lifestyle modifications and anti-hypertensive medications. During treatment with VSPi, blood pressures should be closely monitored, and hypertension should be treated with first-line medications according to the Joint National Committee (JNC 8) guidelines (Table 2).
Table 2: Proposed Mechanisms and Management of Common Vascular Toxicities
| Thromboembolism | CAD/MI | PAD | Hypertension | PAH | Arterial Vasospasm | |||
| Proposed Mechanism(s) |
|
|
|
|
|
|||
| Management* | Step 1 |
|
||||||
| Screening |
|
|
|
|
|
|
||
| Diagnosis |
|
|
|
|
|
|
||
| Treatment |
|
|
|
|
|
|
||
5-FU, 5-fluorouracil; ABI, ankle-brachial index; ACEi, angiotensin-converting enzyme inhibitors; ACS, acute coronary syndrome; ARB, angiotensin receptor blockers; ASCVD, atherosclerotic cardiovascular disease; CACs, coronary artery calcium score; CAD, coronary artery disease; CCB, calcium channel blockers; cCTA, coronary CT angiography; CHF, congestive heart failure; CPET, cardiopulmonary exercise testing; CVD, cardiovascular disease; ERAs, endothelin-receptor antagonists; HTN, hypertension; ICIs, immune checkpoint inhibitors; LE, lower extremity; MI, myocardial infarction; PAD, peripheral artery disease; PAH, pulmonary arterial hypertension; PCI, percutaneous coronary intervention; PDE-5, phosphodiesterase-5; PND, paroxysmal nocturnal dyspnea; RV, right ventricle; RVH, right ventricular hypertrophy; TKIs, tyrosine kinase inhibitors; UE, upper extremity; VEGF, vascular endothelial growth factor; WMA, wall motion abnormalities
Venous and Arterial Thromboembolism
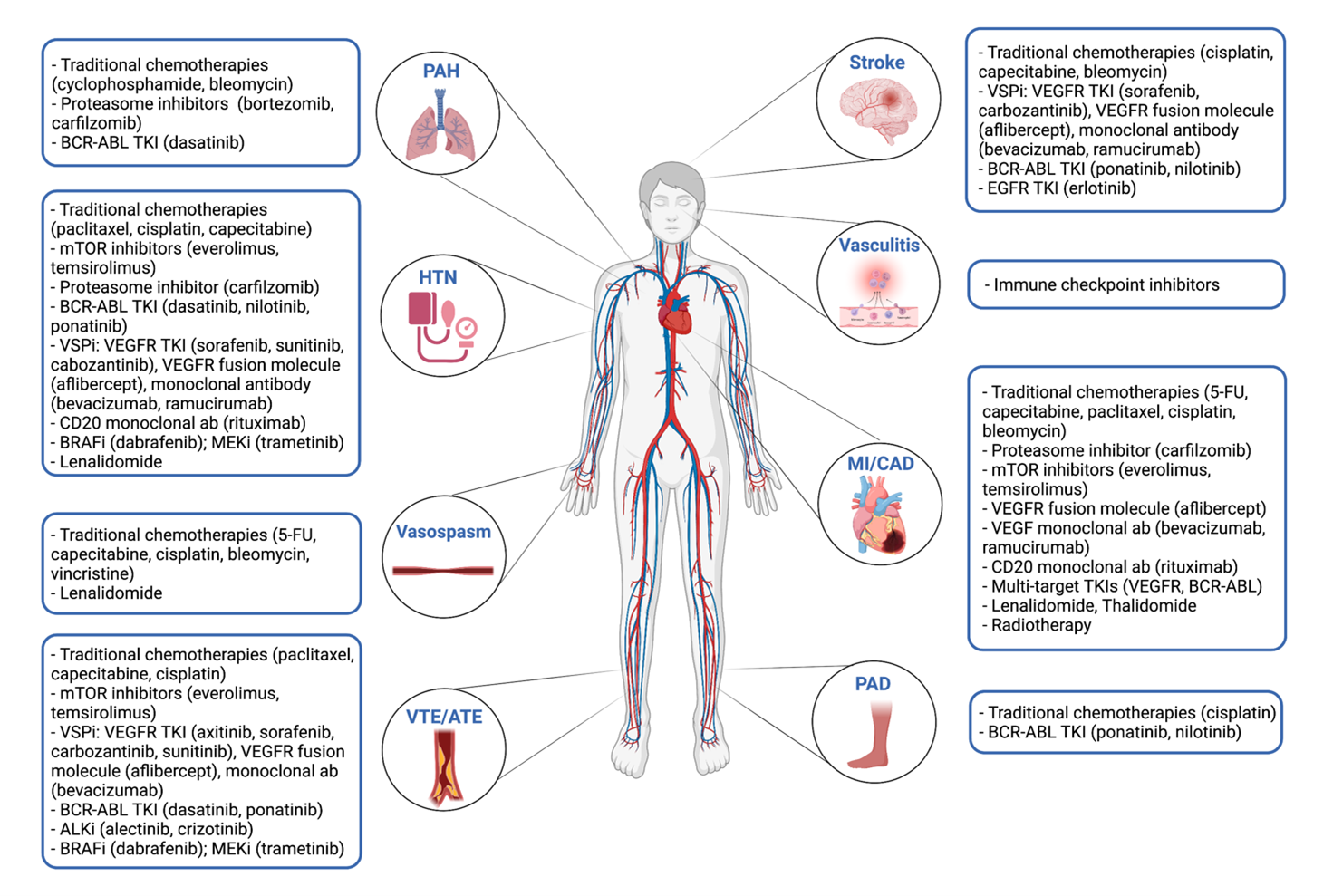
Thromboembolism is prevalent in cancer patients and is associated with high rates of morbidity and mortality.11 Cancer treatment can further increase the incidence of venous- (VTE) and arterial thromboembolism (ATE), including thrombotic events such as myocardial ischemia/infarction (MI) (Figure 1). The mechanisms of increased thrombosis due to cancer therapies are proposed to include endothelial activation, endothelial cytotoxicity, platelet activation, and reduced anticoagulant activity. Here, we will focus on targeted therapies and immunotherapies.
Though TKIs result in potent antineoplastic effects by blocking downstream signaling pathways of their targets (e.g., VEGFR, platelet derived growth factor receptor [PDGFR]), their use is associated with an increased risk of thromboembolism (Table 1). For example, sunitinib and sorafenib are associated with a three-fold increase in the risk of ATE.12 Additional VEGFR-TKIs (e.g., pazopanib, vandetanib, axitinib, etc.) are associated with an increased risk of ATE and thrombosis (1.4% vs. 0.5%, OR = 2.26), with MI being the most common.13 The proposed mechanisms include endothelial cell dysfunction and altered vasodilator homeostasis.4 Bevacizumab also increases the risk of both VTE and ATE.14 Indeed, bevacizumab almost doubles the risk of ATE in cancer patients, in part due to increased platelet activation.15-18
BCR-ABL TKIs are currently the standard of care for chronic myeloid leukemia (CML). Second-generation TKIs (nilotinib, dasatinib, ponatinib) have a greater binding affinity for BCR-ABL1 and are more efficacious compared to first-generation TKI (imatinib).19-21 Notably, patients on newer generation TKIs are three times more likely to develop ATE compared to those treated with imatinib.22,23 Preclinical studies suggest that platelet activation is the primary mechanism of ponatinib-associated ATE.24,25
Immune checkpoint inhibitors (ICIs) are increasingly being used as first-line cancer therapies.26,27 ICIs are monoclonal antibodies that impair tumor escape mechanisms by targeting immune checkpoints, such as CTLA-4, PD-1, and PD-L1, among others (e.g., LAG3). Though ATE and VTE have been reported in patients taking ICI, incidence rates are unclear due to lack of systematic toxicity monitoring.28-30 A recent single center retrospective study reported a cumulative incidence of 12.9% (VTE) and 0.6% (ATE) in patients treated with ICIs but a control group was lacking.31 Proposed mechanisms include vasculitis and vascular thrombotic events.32
The management of thromboembolism in cancer patients should be individualized based on bleeding and clotting risks. Though anticoagulation is the most common treatment, fibrinolysis, antiplatelet therapy, and mechanical thrombectomy can be considered on an individualized basis.33 The most recent ASCO (American Society of Clinical Oncology) guidelines do not recommend routine thromboprophylaxis for all cancer patients, though can be considered in high-risk patients (Table 2).34
Atherosclerosis and Peripheral Artery Disease
Peripheral artery disease (PAD) is characterized by progressive atherosclerosis and stenosis of large and medium-sized arteries. PAD commonly affects the lower extremities, resulting in claudication.35 Various cancer therapies have been shown to accelerate atherosclerosis and PAD, especially in patients with underlying CVD risk factors (Figure 1).
PAD is associated with second-generation BCR-ABL TKIs (e.g. nilotinib, ponatinib). Notably, imatinib has a favorable CV side effect profile and may even reduce the risk of PAD.36 Compared to imatinib, nilotinib use is associated with increased risk of pathological ankle-brachial index (ABI) values (RR = 10.3; 95% CI 2.3-61.5).37 Cases of nilotinib-associated PAD can be severe and rapidly progressive, sometimes requiring angioplasty or surgical revascularization.38-40 Ponatinib is also associated with advanced atherosclerosis, including acute MI.41 Recent meta-analyses have confirmed the increased risk of arterial vascular events (e.g. PAD, MI, CVD) with both nilotinib and ponatinib.42,43 Potential mechanisms are summarized in Table 2.
Though clinical reports of accelerated atherosclerosis or PAD in ICIs are limited, pre-clinical studies have demonstrated that genetic deficiency of PD-L1, PD-L2, or PD1 increase inflammatory cell infiltration in atherosclerotic plaques, suggesting a potential link between ICIs and atherosclerosis.44,45 It is thought that ICIs inhibit critical negative regulators of atherosclerosis.32 A recent single-center study showed that patients treated with ICIs are 3.3 times more likely to have an atherosclerotic CV event, defined as MI, coronary revascularization, and ischemic stroke, compared to matched controls over years.46
Management of atherosclerotic disease in cancer patients should be individualized based on risk factors and baseline CV disease. Patients with high CVD risk profiles are more vulnerable to vascular toxicities with TKIs compared to those with low risk profiles.47 Risk factors (e.g., obesity, smoking, diabetes, hypertension, hypercholesterolemia) should be assessed and optimized in patients prescribed BCR-ABL inhibitors. CV and metabolic parameters, including ABI measurements, should be monitored regularly. In patients taking nilotinib or ponatinib with high-grade PAD (i.e., severe claudication plus abnormal ABI or imaging), the TKI should be replaced with another if possible.48
Pulmonary Hypertension
Pulmonary hypertension (PH) is defined by a mean pulmonary artery pressure (mPAP) >20mmHg measured during right heart catheterization (RHC).49 Group 1 PH or pulmonary arterial hypertension (PAH) includes drug-induced, idiopathic, and toxin-induced, and results from uncontrolled growth of endothelial and smooth-muscle cells in the pulmonary vasculature.50,51 Untreated PH can lead to increased pulmonary vascular resistance (PVR), RV hypertrophy and remodeling, culminating in RV failure.
Dasatinib (second-generation BCL-ABL TKI) has been associated with reversible PAH.52-56 Though the incidence of dasatinib-associated PAH was initially estimated to be 0.45%,57 recent studies report an incidence rate of up to 5%.58-60 However, this is likely higher due to under-diagnosis of subclinical PH, as well as recently updated diagnostic criteria (i.e.., mPAP >20mmHg rather than ≥25mmHg). Though cessation of dasatinib typically decreases mPAP, long-term follow up data showed that one-third of patients had persistently elevated mPAP and PVR.61 The proposed mechanisms of dasatinib-induced PAH are summarized in Table 2.
Management of PH in cancer patients is based on guidelines in the general population.62,63 Prior to initiation of dasatinib, patients should be evaluated for signs of underlying cardiopulmonary disease. Patients who develop dyspnea and symptoms of RV dysfunction (e.g., peripheral edema), should be evaluated with electrocardiogram (ECG) and transthoracic echocardiogram (TTE). If PAH is suspected, RHC should be considered. Once PH is confirmed, the culprit drug should be discontinued, and pulmonary vasodilators initiated.
Coronary Artery Vasospasm
Coronary artery vasospasm typically presents with angina, troponin elevation, and ischemic ECG changes. Traditional chemotherapy agents, specifically 5-fluorouracil (5-FU) and capecitabine (prodrug of 5-FU), have the highest risk of vasospasm.64,65 Patients with underlying coronary artery disease or pre-existing endothelial dysfunction are at higher risk of coronary vasospasm.66 Pharmacogenetic variants in DPYD (dihydropyrimidine dehydrogenase) and TYMS (thymidylate synthase) are associated with an increased risk of high grade toxicity.67 Cases of coronary vasospasm have also been reported with other therapies (e.g., paclitaxel, bevacizumab, sorafenib, radiotherapy) (Figure 1). Management recommendations are summarized in Table 2.
Figure 1: Common Vascular Toxicities Associated with Cancer Therapies
Figure created with biorender.com. Courtesy of Song EJ, Baik AH.
References
- Touyz RM, Lang NN, Herrmann J, van den Meiracker AH, Danser AHJ. Recent advances in hypertension and cardiovascular toxicities with vascular endothelial growth factor inhibition. Hypertension 2017;70:220-26.
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 2004;25:581-611.
- Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17:611-25.
- Baik AH. Hypoxia signaling and oxygen metabolism in cardio-oncology. J Mol Cell Cardiol 2021;165:64-75.
- Soria JC, DeBraud F, Bahleda R, et al. Phase I/IIa study evaluating the safety, efficacy, pharmacokinetics, and pharmacodynamics of lucitanib in advanced solid tumors. Ann Oncol 2014;25:2244-51.
- Qi WX, He AN, Shen Z, Yao Y. Incidence and risk of hypertension with a novel multi-targeted kinase inhibitor axitinib in cancer patients: a systematic review and meta-analysis. Br J Clin Pharmacol 2013;76:348-57.
- Izzedine H, Ederhy S, Goldwasser F, et al. Management of hypertension in angiogenesis inhibitor-treated patients. Ann Oncol 2009;20:807-15.
- Hamnvik OPR, Choueiri TK, Turchin A, et al. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer 2015;121:311-19.
- Wicki A, Hermann F, Prêtre V, et al. Pre-existing antihypertensive treatment predicts early increase in blood pressure during bevacizumab therapy: the Prospective AVALUE Cohort study. Oncol Res Treat 2014;37:230-36.
- Pandey AK, Singhi EK, Arroyo JP, et al. Mechanisms of VEGF-inhibitor associated hypertension and vascular disease. Hypertension 2018;71:e1-e8.
- Streiff MB, Holmstrom B, Angelini D, et al. NCCN Guidelines insights: cancer-associated venous thromboembolic disease, Version 2.2018. J Natl Compr Cancer Netw 2018;16:1289-1303.
- Choueiri TK, Schutz FAB, Je Y, Rosenberg JE, Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol 2010;28:2280-85.
- Qi WX, Shen Z, Tang LN, Yao Y. Risk of arterial thromboembolic events with vascular endothelial growth factor receptor tyrosine kinase inhibitors: an up-to-date meta-analysis. Crit Rev Oncol Hematol 2014;92:71-82.
- Patel P, Srinivas S. Toxicities of targeted agents in advanced renal cell carcinoma. Curr Clin Pharmacol 2011;6:181-88.
- Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst 2007;99:1232-39.
- Schutz FAB, Je Y, Azzi GR, Nguyen PL, Choueiri TK. Bevacizumab increases the risk of arterial ischemia: a large study in cancer patients with a focus on different subgroup outcomes. Ann Oncol 2011;22:1404-12.
- Ranpura V, Hapani S, Chuang J, Wu S. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta-analysis of randomized controlled trials. Acta Oncol 2010;49:287-97.
- Meyer T, Robles-Carrillo L, Robson T, et al. Bevacizumab immune complexes activate platelets and induce thrombosis in FCGR2A transgenic mice. J Thromb Haemost 2009;7:171-81.
- An X, Tiwari AK, Sun Y, Ding PR, Ashby CR Jr., Chen ZS. BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: a review. Leuk Res 2010;34:1255-68.
- Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 2010;362:2251-59.
- Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010;362:2260-70.
- Douxfils J, Haguet H, Mullier F, Chatelain C, Graux C, Dogné JM. Association between BCR-ABL tyrosine kinase inhibitors for chronic myeloid leukemia and cardiovascular events, major molecular response, and overall survival: a systematic review and meta-analysis. JAMA Oncol 2016;2:625-32.
- Haguet H, Douxfils J, Mullier F, Chatelain C, Graux C, Dogné JM. Risk of arterial and venous occlusive events in chronic myeloid leukemia patients treated with new generation BCR-ABL tyrosine kinase inhibitors: a systematic review and meta-analysis. Expert Opin Drug Saf 2017;16:5-12.
- Hamadi A, Grigg AP, Dobie G, et al. Ponatinib tyrosine kinase inhibitor induces a thromboinflammatory response. Thromb Haemost 2019;119:1112-23.
- Merkulova A, Mitchell SC, Stavrou EX, Forbes GL, Schmaier AH. Ponatinib treatment promotes arterial thrombosis and hyperactive platelets. Blood Adv. 2019;3:2312-16.
- Zhang L, Reynolds KL, Lyon AR, Palaskas N, Neilan TG. The evolving immunotherapy landscape and the epidemiology, diagnosis, and management of cardiotoxicity: JACC: CardioOncology Primer. JACC CardioOncol 2021;3:35-47.
- Baik AH, Tsai KK, Oh DY, Aras MA. Mechanisms and clinical manifestations of cardiovascular toxicities associated with immune checkpoint inhibitors. Clin Sci (Lond) 2021;135:703-24.
- Boutros C, Scoazec JY, Mateus C, Routier E, Roy S, Robert C. Arterial thrombosis and anti-PD-1 blockade. Eur J Cancer 2018;91:164-66.
- Kunimasa K, Nishino K, Kimura M, et al. Pembrolizumab-induced acute thrombosis: a case report. Medicine (Baltimore) 2018;97;e10772.
- Tsukamoto J, Monteiro M, Vale S, et al. Thromboembolic events related to treatment with checkpoint inhibitors: report of two cases. Case Rep Oncol 2018;11:648-53.
- Moik F, Chan WSE, Wiedemann S, et al. Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood 2021;137:1669-78.
- Budnik I, Brill A. Immune actors in deep vein thrombosis initiation. Trends Immunol 2018;39:610-23.
- Herrmann J, Yang EH, Iliescu CA, et al. Vascular toxicities of cancer therapies: the old and the new--an evolving avenue. Circulation 2016;133:1272-89.
- Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO Clinical Practice Guideline update. J Clin Oncol 2020;38:496-520.
- Shu J, Santulli G. Update on peripheral artery disease: epidemiology and evidence-based facts. Atherosclerosis 2018;275:379-81.
- Giles FJ, Mauro MJ, Hong F, et al. Rates of peripheral arterial occlusive disease in patients with chronic myeloid leukemia in the chronic phase treated with imatinib, nilotinib, or non-tyrosine kinase therapy: a retrospective cohort analysis. Leukemia 2013;27:1310-15.
- Kim TD, Rea D, Schwarz M, et al. Peripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or imatinib. Leukemia 2013;27:1316-21.
- Le Coutre P, Rea D, Abruzzese E, et al. Severe peripheral arterial disease during nilotinib therapy. J Natl Cancer Inst 2011;103:1347-48.
- Aichberger KJ, Herndlhofer S, Schernthaner GH, et al. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am J Hematol 2011;86:533-39.
- Tefferi A, Letendre L. Nilotinib treatment-associated peripheral artery disease and sudden death: yet another reason to stick to imatinib as front-line therapy for chronic myelogenous leukemia. Am J Hematol 2011;86:610-11.
- Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome–positive leukemias. N Engl J Med 2013;369:1783-96.
- Chai-Adisaksopha C, Lam W, Hillis C. Major arterial events in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: a meta-analysis. Leuk Lymphoma 2016;57:1300-10.
- Douxfils J, Haguet H, Mullier F, Chatelain C, Graux C, Dogné JM. Association between BCR-ABL tyrosine kinase inhibitors for chronic myeloid leukemia and cardiovascular events, major molecular response, and overall survival: a systematic review and meta-analysis. JAMA Oncol 2016;2:625-32.
- Bu DX, Tarrio M, Maganto-Garcia E, et al. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler Thromb Vasc Biol 2011;31:1100-07.
- Gotsman I, Grabie N, Dacosta R, Sukhova G, Sharpe A, Lichtman AH. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest 2007;117:2974-82.
- Drobni ZD, Alvi RM, Taron J, et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation 2020;142:2299-2311.
- Rea D, Mirault T, Raffoux E, et al. Usefulness of the 2012 European CVD risk assessment model to identify patients at high risk of cardiovascular events during nilotinib therapy in chronic myeloid leukemia. Leukemia 2015;29:1206-09.
- Pasvolsky O, Leader A, Iakobishvili Z, Wasserstrum Y, Kornowski R, Raanani P. Tyrosine kinase inhibitor associated vascular toxicity in chronic myeloid leukemia. Cardiooncology 2015;1:1-10.
- Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913.
- Hassoun PM. Pulmonary arterial hypertension. N Engl J Med 2021;385:2361-76.
- Wagenvoort CA. The pathology of primary pulmonary hypertension. J Pathol 1970;101:Pi.
- Rasheed W, Flaim B, Seymour JF. Reversible severe pulmonary hypertension secondary to dasatinib in a patient with chronic myeloid leukemia. Leuk Res 2009;33:861-64.
- Mattei D, Feola M, Orzan F, Mordini N, Rapezzi D, Gallamini A. Reversible dasatinib-induced pulmonary arterial hypertension and right ventricle failure in a previously allografted CML patient. Bone Marrow Transplant 2009;43:967-68.
- Orlandi EM, Rocca B, Pazzano AS, Ghio S. Reversible pulmonary arterial hypertension likely related to long-term, low-dose dasatinib treatment for chronic myeloid leukaemia. Leuk Res 2012;36:e4-6.
- Dumitrescu D, Seck C, Ten Freyhaus H, Gerhardt F, Erdmann E, Rosenkranz S. Fully reversible pulmonary arterial hypertension associated with dasatinib treatment for chronic myeloid leukaemia. Eur Respir J 2011;38:218-20.
- Hennigs JK, Keller G, Baumann HJ, et al. Multi tyrosine kinase inhibitor dasatinib as novel cause of severe pre-capillary pulmonary hypertension? BMC Pulm Med 2011;11:30.
- Montani D, Bergot E, Günther S, et al. Pulmonary arterial hypertension in patients treated by dasatinib. Circulation 2012;125:2128-37.
- Shah NP, Rousselot P, Schiffer C, et al. Dasatinib in imatinib-resistant or -intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180-034. Am J Hematol 2016;91:869-74.
- Shah NP, Wallis N, Farber HW, et al. Clinical features of pulmonary arterial hypertension in patients receiving dasatinib. Am J Hematol 2015;90:1060-64.
- Fox LC, Cummins KD, Costello B, et al. The incidence and natural history of dasatinib complications in the treatment of chronic myeloid leukemia. Blood Adv 2017;1:802-11.
- Weatherald J, Chaumais MC, Savale L, et al. Long-term outcomes of dasatinib-induced pulmonary arterial hypertension: a population-based study. Eur Respir J 2017;50:1700217.
- McLaughlin VV, Shah SJ, Souza R, Humbert M. Management of pulmonary arterial hypertension. J Am Coll Cardiol 2015;65:1976-97.
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2015;46:903-75.
- Kleiman NS, Lehane DE, Geyer CE Jr, Pratt CM, Young JB. Prinzmetal's angina during 5-fluorouracil chemotherapy. Am J Med 1987;82:566-68.
- Schnetzler B, Popova N, Collao Lamb C, Sappino AP. Coronary spasm induced by capecitabine. Ann Oncol 2001;12:723-24.
- Meyer CC, Calis KA, Burke LB, Walawander CA, Grasela TH. Symptomatic Cardiotoxicity Associated with 5-Fluorouracil. Pharmacotherapy 1997;17:729-36.
- Loganayagam A, Arenas Hernandez M, Corrigan A, et al. Pharmacogenetic variants in the DPYD, TYMS, CDA and MTHFR genes are clinically significant predictors of fluoropyrimidine toxicity. Br J Cancer 2013;108:2505-15.
- Ramcharan KS, Lip GYH, Stonelake PS, Blann AD. Effect of standard chemotherapy and antiangiogenic therapy on plasma markers and endothelial cells in colorectal cancer. Br J Cancer 2014;111:1742-49.
- Folco EJ, Mawson TL, Vromman A, et al. Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1α and cathepsin G. Arterioscler Thromb Vasc Biol 2018;38:1901-12.
- Gover-Proaktor A, Granot G, Pasmanik-Chor M, et al. Bosutinib, dasatinib, imatinib, nilotinib, and ponatinib differentially affect the vascular molecular pathways and functionality of human endothelial cells. Leuk Lymphoma 2019;60:189-99.
- Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol 1996;27:766-73.
- Yang EH, Marmagkiolis K, Balanescu DV, et al. Radiation-induced vascular disease—A State-of-the-Art Review. Front Cardiovasc Med 2021;8:652761.
- Guignabert C, Phan C, Seferian A, et al. Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. J Clin Invest 2016;126:3207-18.
- Ryan JJ. Tyrosine kinase inhibitors in pulmonary vascular disease. JACC Basic Transl Sci 2016;1:684-86.
- Mosseri M, Fingert HJ, Varticovski L, Chokshi S, Isner JM. In vitro evidence that myocardial ischemia resulting from 5-fluorouracil chemotherapy is due to protein kinase C-mediated vasoconstriction of vascular smooth muscle. Cancer Res 1993;53;3028-33.
- Montazeri K, Unitt C, Foody JM, Harris JR, Partridge AH, Moslehi J. ABCDE steps to prevent heart disease in breast cancer survivors. Circulation 2014;130:e157-e159.
- Aboumsallem JP, Moslehi J, de Boer RA. Reverse cardio-oncology: cancer development in patients with cardiovascular disease. J Am Heart Assoc 2020;9:e013754.
- Breccia M, Molica M, Zacheo I, Serrao A, Alimena G. Application of systematic coronary risk evaluation chart to identify chronic myeloid leukemia patients at risk of cardiovascular diseases during nilotinib treatment. Ann Hematol 2015;94:393-97.
- Small HY, Montezano AC, Rios FJ, Savoia C, Touyz RM. Hypertension due to antiangiogenic cancer therapy with vascular endothelial growth factor inhibitors: understanding and managing a new syndrome. Can J Cardiol 2014;30:534-43.
Clinical Topics: Anticoagulation Management, Cardiac Surgery, Cardio-Oncology, Cardiovascular Care Team, Diabetes and Cardiometabolic Disease, Dyslipidemia, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Prevention, Pulmonary Hypertension and Venous Thromboembolism, Stable Ischemic Heart Disease, Vascular Medicine, Atherosclerotic Disease (CAD/PAD), Anticoagulation Management and Venothromboembolism, Aortic Surgery, Cardiac Surgery and Heart Failure, Cardiac Surgery and SIHD, Homozygous Familial Hypercholesterolemia, Novel Agents, Statins, Heart Failure and Cardiac Biomarkers, Pulmonary Hypertension, Interventions and Coronary Artery Disease, Interventions and Vascular Medicine, Hypertension, Smoking, Chronic Angina
Keywords: Dasatinib, Ankle Brachial Index, Angioplasty, Antihypertensive Agents, Anticoagulants, Atherosclerosis, Axitinib, B7-H1 Antigen, Bevacizumab, Blood Pressure, Brain Diseases, Brain Ischemia, Cardiac Catheterization, Capecitabine, Cardiovascular Diseases, Classification, Constriction, Pathologic, Control Groups, Coronary Artery Disease, Coronary Vasospasm, CTLA-4 Antigen, Diabetes Mellitus, Dihydrouracil Dehydrogenase (NADP), Doxorubicin, Dyspnea, Rabeprazole, Edema, Electrocardiography, Endothelial Cells, Fibrinolysis, Follow-Up Studies, Goals, Homeostasis, Hypercholesterolemia, Hyperlipidemias, Hypertension, Hypertension, Pulmonary, Hypertrophy, Imatinib Mesylate, Immune Checkpoint Inhibitors, Immune Checkpoint Inhibitors, Immunotherapy, Infarction, Ischemic Stroke, Leukemia, Myelogenous, Chronic, BCR-ABL Positive, Life Style, Lower Extremity, Medical Oncology, Muscle Cells, Myocardial Ischemia, Neoplasms, Obesity, Paclitaxel, Peripheral Arterial Disease, Pharmacogenomic Variants, Plaque, Atherosclerotic, Platelet Activation, Platelet Aggregation Inhibitors, Prodrugs, Programmed Cell Death 1 Receptor, Protein Kinase Inhibitors, Pulmonary Arterial Hypertension, Pulmonary Artery, Receptors, Platelet-Derived Growth Factor, Receptors, Vascular Endothelial Growth Factor, Retrospective Studies, Risk Factors, Signal Transduction, Smoking, Sorafenib, Standard of Care, Stroke, Sunitinib, Thrombectomy, Thrombosis, Thymidylate Synthase, Tobacco Use, Troponin, Tumor Escape, Vascular Endothelial Growth Factor A, Vascular Endothelial Growth Factor A, Vascular Resistance, Vasculitis, Vasodilator Agents, Venous Thromboembolism
< Back to Listings