Catheter Ablation of Ventricular Tachycardia After the First ICD Shock: Impact on Mortality and Heart Failure Hospitalization
Quick Takes
- Catheter ablation performed after the first implantable cardioverter defibrillator shock reduced the risk of death or worsening heart failure hospitalization.
- Catheter ablation for ventricular tachycardia may be considered after the first implantable cardioverter defibrillator shock in patients with ischemic or nonischemic cardiomyopathy.
- Antitachycardia pacing predicted the occurrence of appropriate implantable cardioverter defibrillator shocks. Therapeutic strategies should aim at reducing the burden of antitachycardia pacing treatments.
Based on the current expert consensus statement,1 referral for catheter ablation is usually prompted by failure of antiarrhythmic drugs (AAD) to control ventricular tachycardia (VT) episodes both in ischemic (ICM) and non-ischemic cardiomyopathy (NICM). A new timing for catheter ablation was introduced in the consensus with a class IIb indication for catheter ablation in patients with ICM and an implantable cardioverter-defibrillator (ICD) after the first episode of monomorphic VT. This new indication stemmed from retrospective data2,3 showing that an earlier ablation of VT is associated with a lower rate of VT recurrence, although without a survival benefit. Prospective randomized trials on the timing of catheter ablation of VT have focused on the two ends of the spectrum of the natural history of the arrhythmia. Prophylactic catheter ablation4-8 at the time of ICD implantation on one side and catheter ablation in patients' refractory to AADs on the other.9 Prophylactic catheter ablation has shown to reduce VT recurrences in most trials but with no benefits in terms of mortality unless in composite endpoints that included ICD treatments.8 Considering its unfavorable cost-benefit ratio, it is unlikely that such an early timing of ablation will be applied in clinical practice. On the other hand, we learned that in relatively advanced disease patients, catheter ablation was superior to escalated drug therapy after failing an AAD in the composite endpoint of death, VT storm, or ICD shock, but with no improvement with respect to mortality alone.9 New randomized controlled trials (RCTs) need to address when to perform ablation between these two extreme timings, and evaluate whether there is a mortality benefit of an early referral. The scope of the PARTITA trial by Della Bella et al. published in Circulation10 is to evaluate the benefit of ablation after the first ICD shock in terms of mortality and worsening heart failure (HF) compared to a deferred strategy.
This is a two-phase, prospective multicenter RCT, which enrolled 517 patients with ischemic or nonischemic dilated cardiomyopathy and indication to an ICD. In phase A patients were followed using a home monitoring system to evaluate their arrhythmic burden until the first appropriate ICD shock for monomorphic VT. Forty-seven were randomly assigned 1:1 in phase B to immediate ablation or continuation of standard therapy without undergoing any ablation procedure until an electrical storm (ES) episode occurred. Amiodarone was not allowed except for documented atrial arrhythmias or as a bridge to ablation in case of ES. ICD programming was standardized, including long detection intervals. A uniform mapping and ablation protocol was adopted with the procedural endpoint of late potential abolition and VT noninducibility.
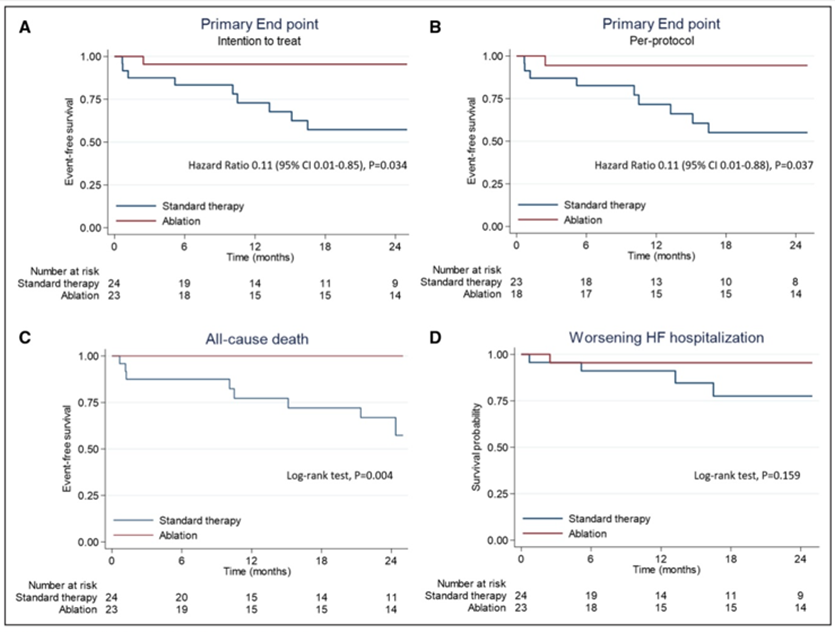
The primary end point was a composite of death from any cause or hospitalization for worsening HF. The trial was interrupted following the first interim analysis. Of the 517 patients enrolled in phase A, 154 (30%) had VT, 56 (11%) received an appropriate shock, and 47 agreed to participate in phase B. After a 2-year follow up the composite primary end point of death or worsening HF hospitalization occurred in significantly fewer patients in the ablation group as compared with standard therapy (4% vs. 42%; logrank P=0.010) (Figure 1), meeting the criteria for study interruption. Furthermore the proportions of patients with all-cause death (0% vs. 33%; P=0.004) and recurrent VT with shocks (9% vs. 42%; P=0.039) were significantly lower in the ablation group. Of note, the overall rate of ICD shocks was low (0.31 per patient-month), and it was estimated that every antitachycardia pacing (ATP) intervention increased the risk of shock by 4%.
Figure 1
A. Survival free from the primary end point—death at any time or worsening heart failure (HF)—among patients treated with catheter ablation or standard therapy at the intention-to-treat analysis. B. Survival free from the primary end point at per protocol analysis. C. Rates of death. D. Rates of worsening HF. Reprinted with permission from Della Bella P, Baratto F, Vergara P, et al. Does timing of ventricular tachycardia ablation affect prognosis in patients with an implantable cardioverter defibrillator? Results from the multicenter randomized PARTITA trial. Circulation 2022;Apr 3:[Epub ahead of print].
The PARTITA study is the first prospective trial to demonstrate the benefits of an early referral for ablation after the first ICD shock on the composite end point of death or worsening HF in patients with ICM or NICM. For the first time a prospective trial demonstrated an improvement on survival alone. One possible explanation is the new timing of randomization. Considering ICD shocks are associated with an increase in mortality, they could be a pivotal point for the identification of patients at a higher risk of death. Prophylactic trials possibly included many patients who would not develop arrhythmias and require ICD treatments. This was confirmed in the PARTITA trial where after ICD implantation, the rate of shocks was low and 70% of the population did not have sustained VT episodes during a mean follow-up of 2.4 years in phase A. On the other hand, the study randomized to ablation patients with an active arrhythmia pattern, as documented by the finding that 88% of patients had multiple episodes of VT treated by ATP before the first shock. In addition to the different selection criteria, other trials did not always report strict ICD programming,4 or had drug management fluctuations,6 whereas mapping and ablation strategies were not uniform among centers.5,6 Comparison of catheter ablation versus AAD after ICD therapy is being addressed in two trials, the ongoing VANISH 2 and the recently published SURVIVE-VT trial.11 In the latter, catheter ablation reduced the composite endpoint of cardiovascular death, appropriate ICD shock hospitalization due to HF, or severe treatment-related complications compared to AAD. The results were driven by treatment-related complications in the drug arm where VT recurrences were the most common adverse event.
Future RCTs are needed to confirm a mortality benefit of an earlier referral of catheter ablation; however, it is well known that recruitment of this population is challenging. First, it is difficult to enroll subjects at the right time as they are frequently referred to tertiary centers when AADs have failed, and the disease is more advanced. Second, limited duration of the follow-up period due to costs, comorbidities and advanced age may concur to overall non-cardiac mortality, thus increasing the difficulty of proving a mortality benefit.
In conclusion, the PARTITA trial is an important piece of the puzzle on the benefits of an early referral for VT ablation as it provides evidence that catheter ablation should be considered after the first ICD shock in patients with ICM and NICM. These findings warrant further adequately powered clinical trials to prove a net benefit of VT ablation after a first ICD shock over alternative timings and strategies.
References
- Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Heart Rhythm 2020;17:e2-e154.
- Frankel DS, Mountantonakis SE, Robinson MR, Zado ES, Callans DJ, Marchlinski FE. Ventricular tachycardia ablation remains treatment of last resort in structural heart disease: argument for earlier intervention. J Cardiovasc Electrophysiol 2011;22:1123–28.
- Dinov B, Arya A, Bertagnolli L, et al. Early referral for ablation of scar-related ventricular tachycardia is associated with improved acute and long-term outcomes: results from the Heart Center of Leipzig ventricular tachycardia registry. Circ Arrhythm Electrophysiol 2014;7:1144–51.
- Reddy VY, Reynolds MR, Neuzil P, et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med 2007;357:2657–65.
- Kuck KH, Schaumann A, Eckardt L, et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicenter randomised controlled trial. Lancet 2010;375:31–40.
- Kuck KH, Tilz RR, Deneke, et al. Impact of substrate modification by catheter ablation on implantable cardioverter-defibrillator interventions in patients with unstable ventricular arrhythmias and coronary artery disease: results from the multicenter randomized controlled SMS (Substrate Modification Study). Circ Arrhythm Electrophysiol 2017;10:e004422.
- Willems S, Tilz RR, Steven D, et al. Preventive or deferred ablation of ventricular tachycardia in patients with ischemic cardiomyopathy and implantable defibrillator (BERLIN VT): a multicenter randomized trial. Circulation 2020;141:105767.
- Tung R, Xue Y, Chen M, et al. First-line catheter ablation of monomorphic ventricular tachycardia in cardiomyopathy concurrent with defibrillator implantation: the PAUSE-SCD randomized trial. Circulation 2022;May 4:[Epub ahead of print].
- Sapp JL, Wells GA, Parkash R, et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med 2016;375:111–21.
- Della Bella P, Baratto F, Vergara P, et al. Does timing of ventricular tachycardia ablation affect prognosis in patients with an implantable cardioverter defibrillator? Results from the multicenter randomized PARTITA trial. Circulation 2022;Apr 3:[Epub ahead of print].
- Arenal Á, Ávila P, Jiménez-Candil J, et al. Substrate ablation vs antiarrhythmic drug therapy for symptomatic ventricular tachycardia. J Am Coll Cardiol 2022;79:1441-53.
Clinical Topics: Arrhythmias and Clinical EP, Heart Failure and Cardiomyopathies, Implantable Devices, EP Basic Science, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Acute Heart Failure
Keywords: Anti-Arrhythmia Agents, Prospective Studies, Defibrillators, Implantable, Retrospective Studies, Tachycardia, Ventricular, Catheter Ablation, Cardiomyopathies, Heart Failure, Recurrence, Catheters, Referral and Consultation, Follow-Up Studies, Cardiomyopathy, Dilated, Hospitalization, Adenosine Triphosphate, Randomized Controlled Trials as Topic, Multicenter Studies as Topic, Patient Selection, Random Allocation
< Back to Listings