Feature | What You Need to Know: Guidance For Clinicians on Dobutamine Shortage
This document was developed by the ACC Heart Failure and Transplant Member Section, ACC Cardiovascular Team Section and University of Utah Health.
Contributing authors: Maya Guglin, MD, FACC; Kristen Campbell, PharmD, FACC; Erin Fox, PharmD; Douglas Jennings, PharmD, FACC; Robert Page, PharmD; Kashif Saleem, MD; Dustin Spencer, PharmD; Barbara Wiggins, PharmD, FACC; Craig J. Beavers, PharmD, FACC.
Background
The current dobutamine shortage affects multiple areas in cardiovascular medicine, including diagnostics and therapies. According to manufacturers, the crisis will be relieved in the fall of 2022 and resolved by the end of this year.
A short-acting intravenous sympathomimetic with positive inotropic and mild vasodilatory properties, the dobutamine shortage mostly impacts patients with advanced heart failure (HF) and other low output syndromes such as cardiogenic shock and early postoperative states in patients with low left ventricular ejection fraction (LVEF). Diagnostic applications such as dobutamine stress echocardiography (DSE) are also affected.
The following guidance is recommended until the dobutamine shortage is resolved:
- When possible, use the alternative catecholamine or non-catecholamine inotropes and vasopressors, especially in the areas where there is no strong evidence in favor of dobutamine, such as postoperative management in patients with normal or mildly reduced cardiac output.
- Consider milrinone as the best alternative to dobutamine for low cardiac output conditions, including advanced HF, cardiogenic shock and postoperative states in patients with low LVEF.
- Utilize other diagnostic modalities such as adenosine nuclear stress test or stress CMR for evaluation of ischemia in place of DSEs.
- Utilize other diagnostic modalities, including MRI, PET or SPECT with thallium or technetium in place of DSE to assess myocardial viability.
- No good alternatives are available for low-dose DSE, thus consider prioritizing dobutamine for this use.
Background on Drug Shortages
Cardiovascular drugs have consistently been among the top classes of drugs that have experienced a shortage over the last five years, with injectable cardiovascular agents often being impacted.1 These shortages can impact the delivery of patient care leading to a compromise of or delay in medical care or procedures; an increased potential for medication errors and subsequent patient harm; and increased health care resource utilization as well as cost.2
The factors leading to these drugs shortages are complex and diverse and include, but are not limited to, these factors: a unique market for drug products; manufacturing and quality problems; production delays and lack of capacity; manufacturing business decisions; shortage of active pharmaceutical ingredients and/or raw materials; restricted distribution and allocation of drug products; and inventory practices.2 An overview of the current environment can be viewed in this CBS 60 Minutes episode.
As a result of the CARES Act, the National Academies of Science, Engineering, and Medicine (NASEM) conducted a study and released a framework to build resilience into medical supply chains with the intent of eliminating constant shortages.
Regular updates on drug shortages, along with general management recommendations, are provided by the U.S. Food and Drug Administration (FDA) and the American Society of Health System Pharmacists.
Pfizer and the Dobutamine Shortage
Pfizer is the sole manufacturer of dobutamine in vials and it produces about 30% of dobutamine in flexible containers. We contacted them to obtain current information about the dobutamine shortage.
The supply of dobutamine flexible container products from Pfizer has been limited for several months due to a complex combination of manufacturing challenges, including significant and sustained increases in demand arising from competitor shortages, as well as a delay in obtaining a component supplied by a third party, also caused by an increased demand because of a limited supply and manufacturing delays.
Manufacturing of both dobutamine vial and flexible container presentations is in progress, with next deliveries for dobutamine products currently anticipated in September 2022. To help ensure equitable distribution of inventory, as product becomes available, Pfizer will release limited quantities of product to wholesalers as well as through direct shipments, on a weekly basis. In addition, Pfizer is pursuing an emergency date extension for certain dobutamine lots from the FDA and will be sharing more information as soon as possible.
Pfizer's estimate of full recovery – to fill all backorders and have sufficient inventory available to meet Pfizer's historical demand, in addition to several weeks of safety stock in the distribution centers – is Q4 2022 to Q1 2023, depending on the presentation. However, external factors such as market shortages may cause these estimates to change.
Pharmacological Properties of Dobutamine and Comparison With Other Drugs
Dobutamine is a sympathomimetic amine, which acts on beta-1, beta 2 and alpha-1 adrenergic receptors. The stimulation of these receptors produces a relatively strong additive inotropic effect and a relatively weak chronotropic effect (Table 1). Alpha-1 agonist activity in the vasculature causes vasoconstriction, which balances the beta-2 vasodilatory effect permitting relatively unchanged blood pressure with administration.
Table 1. Comparison of Dobutamine vs. Alternative Vasoactive Drugs
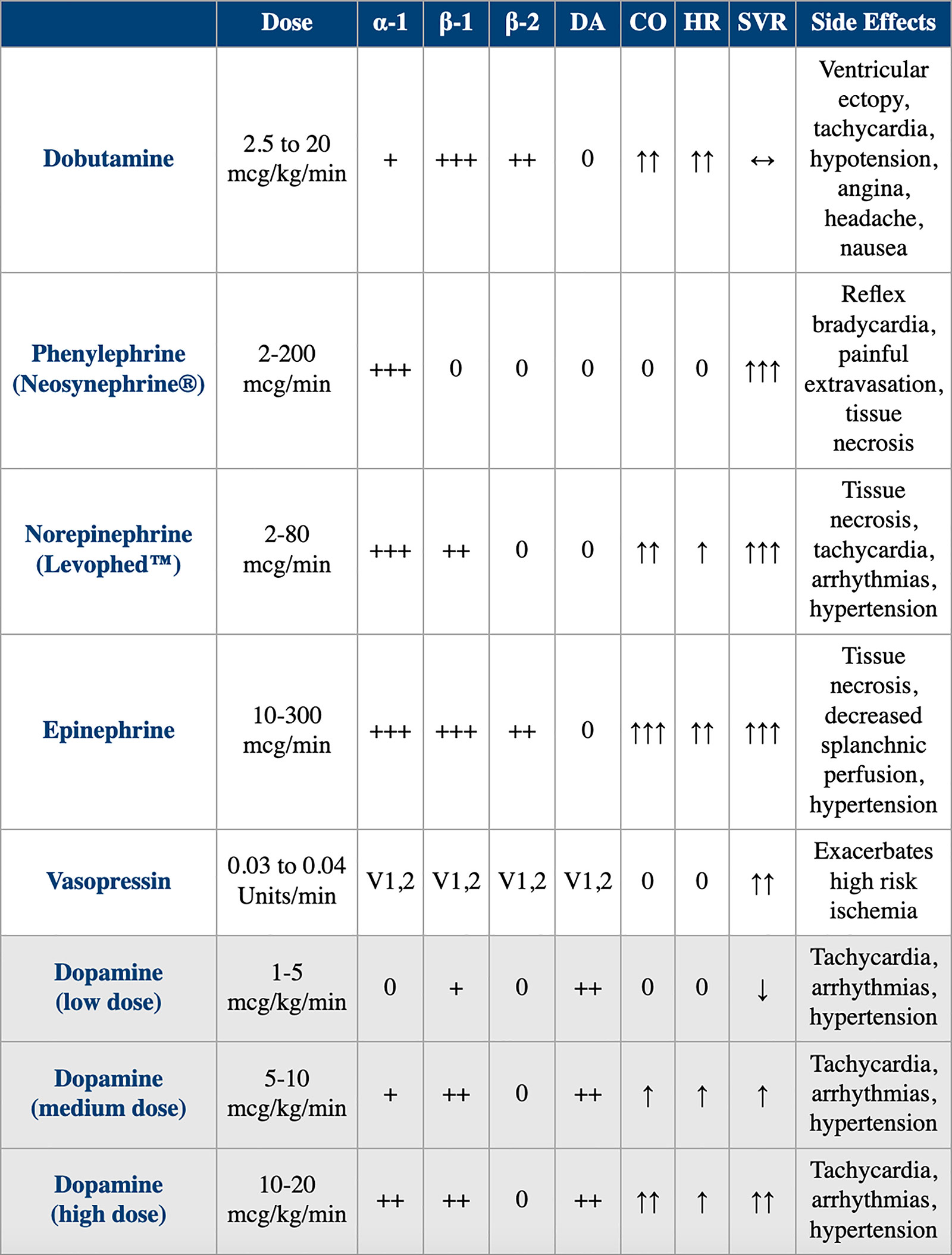 CO, cardiac output; DA, dopamine; HR, heart rate; SVR, systemic vascular resistance; V, vasopressin.
CO, cardiac output; DA, dopamine; HR, heart rate; SVR, systemic vascular resistance; V, vasopressin.
Dobutamine increases myocardial contractility, with accompanying reflex reduction in sympathetic tone. In patients with HF, its use has actually been shown to cause a dose-dependent decrease in plasma norepinephrine. Overall, this leads to an increase in cardiac output by selective augmentation of stroke volume with a decrease in systemic vascular resistance. Because of its adrenergic properties, the use of dobutamine is problematic in patients who take beta-blockers. Tables 2 and 3 provide a pharmacological comparison of dobutamine with milrinone.
Table 2. Pharmacology and Pharmacokinetics of Dobutamine and Milrinone
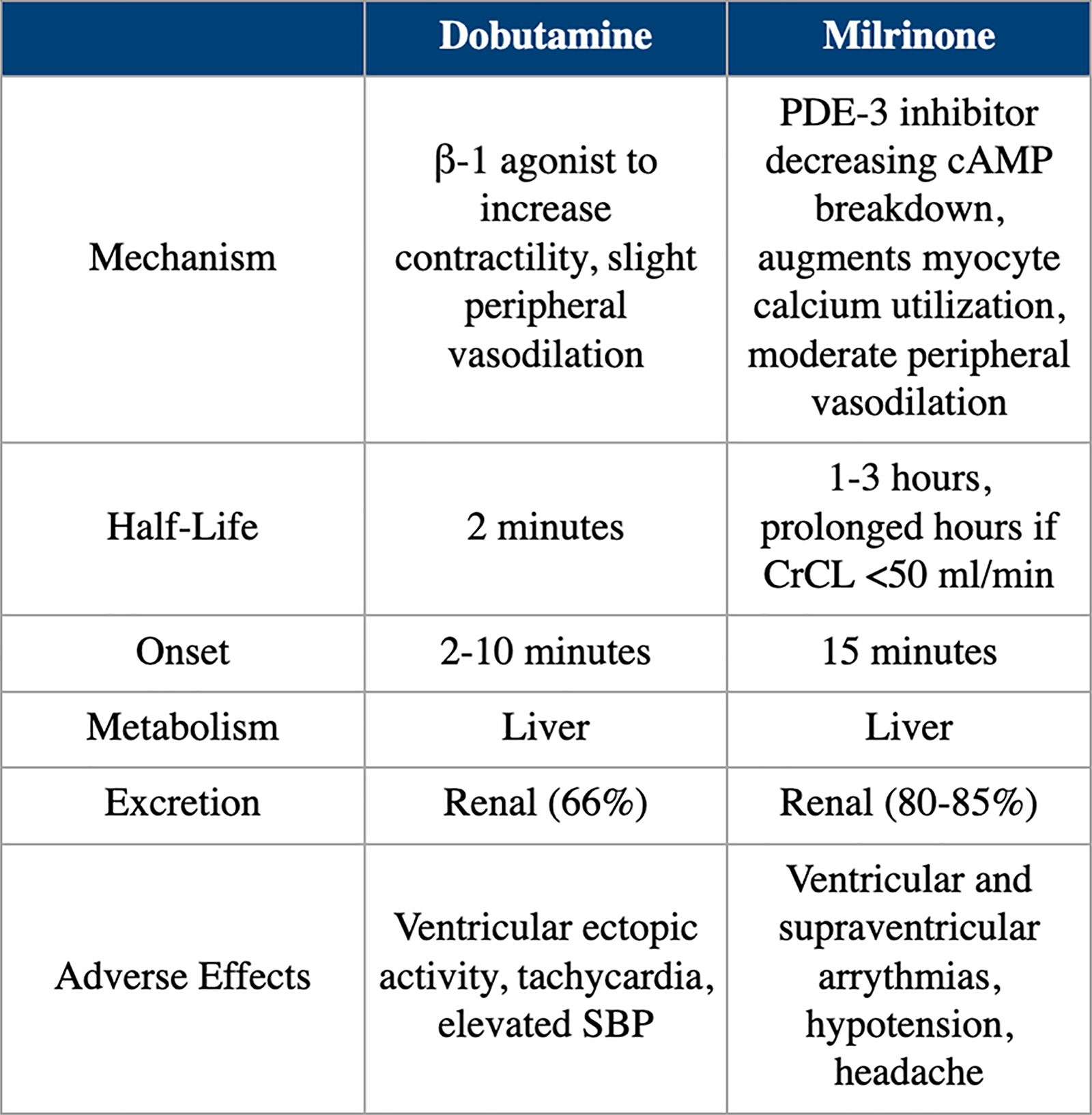 cAMP, cyclic adenosine monophosphate; CrCL, creatine clearance; PDE, phosphodiesterase; SBP, systolic blood pressure.
cAMP, cyclic adenosine monophosphate; CrCL, creatine clearance; PDE, phosphodiesterase; SBP, systolic blood pressure.
Table 3. Hemodynamic Effects of Dobutamine and Milrinone
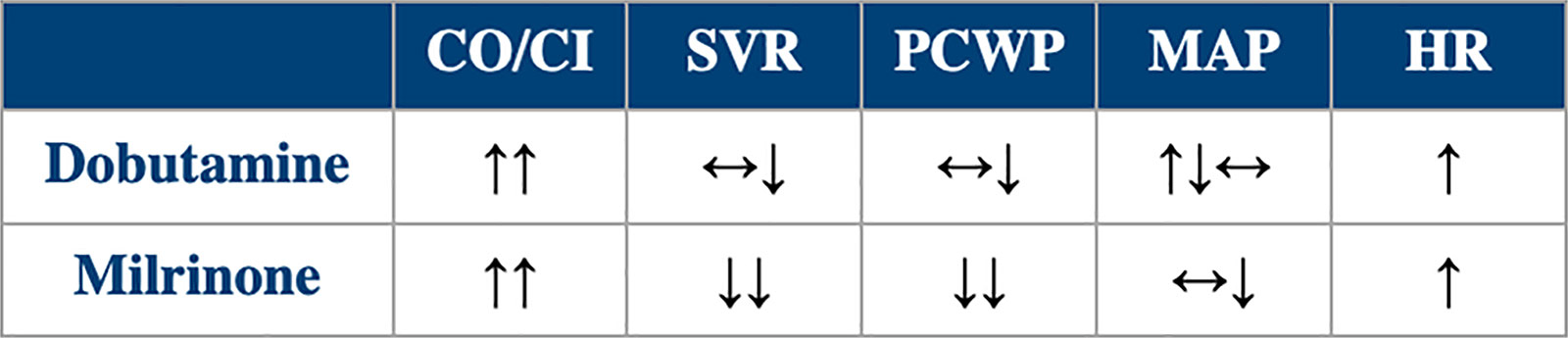 CI, cardiac index; CO, cardiac output; HR, heart rate; MAP, mean arterial pressure; PCWP, pulmonary capillary wedge pressure; SVR, systemic vascular resistance.
CI, cardiac index; CO, cardiac output; HR, heart rate; MAP, mean arterial pressure; PCWP, pulmonary capillary wedge pressure; SVR, systemic vascular resistance.
Clinical Scenarios
Scenario 1. DSE to Evaluate Ischemia in Patients Unable to Exercise
Dobutamine is given in graded doses starting at 5 mcg/kg/min and increased at 3-minute intervals to 10, 20, 30 and 40 mcg/kg/min doses with the goal of achieving an 85% of age-adjusted maximal heart rate.3
Although no pharmacologic agent can adequately substitute for dobutamine (dipyridamole is rarely used because of more frequent side effects), other modalities can be used to evaluate for ischemia and should be used during the dobutamine shortage. These modalities include radionuclide SPECT, real-time myocardial contrast echocardiography and stress CMR.
For detection of ischemia, DSE has a similar sensitivity to nuclear perfusion imaging (SPECT), except for the detection of left main or multivessel coronary artery disease, for which the dobutamine technique is more sensitive. However, DSE has higher specificity.3
Scenario 2. Assessment of Myocardial Viability
DSE is the preferred method to assess myocardial viability.3 Dobutamine is given in incremental doses of 2.5, 5, 7.5 and 10 mcg/kg/min. The assessment of viability is based on a differential response of stunned or hibernated myocardium to the increasing doses of dobutamine.
No other pharmacological agent can replace dobutamine. However, nonpharmacologic modalities can be used, including MRI, PET, or SPECT with thallium, technetium or fluorine deoxyglucose.
Contractile reserve by DSE compares favorably with nonpharmacologic modalities for viability evaluation.
Scenario 3. Low-Dose Dobutamine Echocardiography to Evaluate For Low-Flow Aortic Stenosis
In patients with low LVEF, dobutamine helps to both grade the severity of aortic stenosis and detect LV contractile reserve. Specifically, in patients with aortic pseudostenosis the aortic valve area can increase and the transaortic gradient decrease in response to the effect of dobutamine on cardiac contractility and increased cardiac output.
Dobutamine is given in a starting dose of 2.5-5.0 mcg/kg/min with a gradual increase to a maximal dose of 20 mcg/kg/min.4 For this indication, dobutamine is a unique agent, with no alternative modalities currently existing.
Scenario 4. Postoperative Management of Normal or Mildly Reduced Cardiac Output After Open Heart Surgery
Dobutamine is often used routinely in the early postoperative period after an open-heart surgery when cardiopulmonary bypass is used, such as CABG or valve replacement/repair. There is little evidence guiding the use of multiple vasoactive drugs in this setting. Some studies indicate that catecholamines in general and dobutamine in particular may not be helpful.
In a propensity-matched study, comparing postoperative management with and without catecholamines (90% dobutamine), catecholamines were associated with higher morbidity.5 In another study, postoperative inotrope exposure appeared to be independently associated with increased hospital mortality, even after adjustment for multiple factors including LVEF, perioperative intraaortic balloon pump use, bypass time, reoperation and cardiac index.6
Finally, a randomized controlled trial in patients undergoing cardiac surgery with a normal LVEF, a dobutamine-sparing strategy (in which the use of dobutamine was guided by hemodynamic evidence of low cardiac output associated with signs of inadequate tissue perfusion) was noninferior to an inotrope-to-all strategy (in which all patients received dobutamine).7 This may be the clinical scenario where dobutamine can be utilized less, without compromising patient care, at least until the shortage is resolved.
Scenario 5. Low Cardiac Output Syndrome (Acute and Chronic HF, Cardiogenic Shock)
Patients in a low output state (cardiac index <2.2 L/min/m2), especially combined with pulmonary hypertension and systemic and vascular congestion, uniquely benefit from inotropes. There are only two inotropes currently available in the U.S.: dobutamine and milrinone. Shortages of dobutamine almost invariably will result in some degree of shortage of milrinone, because it is the only viable alternative.
In cardiogenic shock, cardiac output can be increased by a variety of devices for temporary mechanical circulatory support, such as intraaortic balloon pump, percutaneous left ventricular assist device, extracorporeal membrane oxygenation, etc. The shortage of dobutamine should encourage early and aggressive use of such devices.
In the more chronic state of advanced HF, the condition of inotrope dependency, when the patients cannot be weaned off dobutamine or milrinone, the shortage of inotropes creates a real threat, compromising patient care.
Tables 2 and 3 compare the different effects of these two agents. Although most features favor milrinone over dobutamine, their main hemodynamic effects are not too different from each other, but dobutamine is much cheaper. Parenteral inotropes remain an option to help the subset of patients with HF who are refractory to other therapies and are suffering consequences from end-organ hypoperfusion.8
In the postoperative management of patients with low cardiac output, such as right ventricular failure after cardiac transplantation, alternative drugs include milrinone, epinephrine or a combination of these two drugs.
Conclusions
The current shortage of dobutamine creates challenges for patient care in several areas of cardiology. For some diagnostic modalities, such as DSE for risk stratification of coronary artery disease, detection of ischemia or assessment of myocardial viability, dobutamine can be replaced by alternative, mostly nonpharmacological means of testing, although with some loss in sensitivity and specificity. In postoperative care of patients who had surgery on cardiopulmonary bypass but have normal cardiac output, the shortage of dobutamine can create an opportunity to reassess the need for the routine use of vasoactive agents. Besides, there are several other agents that can be used.
It must be noted that there is no alternative to the use of dobutamine for grading the severity of low-flow aortic stenosis. Fortunately, this is a narrow area, and in most cases the test can be postponed by weeks or even months.
For the care of patients with low-output HF, the dobutamine shortage creates a very serious problem. All measures must be taken to provide an uninterrupted supply of dobutamine and milrinone. The ACC and other professional societies should use all available means including advocacy to resolve this shortage.
References
- Fox E. American Society of Health-system Pharmacists Drug Shortage Statistics. Available here. Accessed July 24, 2022.
- American Society of Health-system Pharmacists. ASHP Guidelines on Managing Drug Product Shortages. Am J Health-Syst Pharm 2018;75:1742-50.
- Pellikka PA, Arruda-Olson A, Chaudhry FA, et al. Guidelines for performance, interpretation, and application of stress echocardiography in ischemic heart disease: From the American Society of Echocardiography. Am Soc Echocardiogr 2020;33:1-41.e8.
- Baumgartner H, Hung J, Bermejo J, et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr 2017;30:372-92.
- Fellahi JL, Parienti JJ, Hanouz JL, et al. Perioperative use of dobutamine in cardiac surgery and adverse cardiac outcome: propensity-adjusted analyses. Anesthesiology 2008;108:979-87.
- Shahin J, DeVarennes B, Tse CW, et al. The relationship between inotrope exposure, six-hour postoperative physiological variables, hospital mortality and renal dysfunction in patients undergoing cardiac surgery. Crit Care 2011;7;15:R162
- Franco RA, de Almeida JP, Landoni G, et al. Dobutamine-sparing versus dobutamine-to-all strategy in cardiac surgery: a randomized noninferiority trial. Ann Intensive Care 2021;11:15.
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2022;79:e263-e421.
Clinical Topics: Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Acute Heart Failure, Interventions and Imaging, Computed Tomography, Echocardiography/Ultrasound, Magnetic Resonance Imaging, Nuclear Imaging
Keywords: Sympathomimetics, Dobutamine, Stroke Volume, Milrinone, Receptors, Adrenergic, alpha-1, Technetium, Thallium, United States Food and Drug Administration, Blood Pressure, Cardiac Output, Low, Echocardiography, Stress, Exercise Test, Patient Harm, Pharmacists, Shock, Cardiogenic, Vasoconstriction, Ventricular Function, Left, Medication Errors, Delivery of Health Care, Cardiovascular Agents, Heart Failure, Patient Care, Adenosine, Tomography, Emission-Computed, Single-Photon, Magnetic Resonance Imaging, Cine, Positron Emission Tomography Computed Tomography
< Back to Listings

