NLA 2022 Definition of Statin Intolerance
Quick Takes
- The 2022 NLA scientific statement on statin intolerance provides an updated definition of statin intolerance which now classifies intolerance as either partial or complete, taking into consideration the ability to achieve therapeutic targets on maximally tolerated statin dosing.
- Statin intolerance exists as a spectrum of muscle related symptoms, which may or may not be related to statin therapy directly, and a significant nocebo effect (perceived symptoms).
- In addition to ezetimibe, other novel lipid lowering medications have emerged in recent years which may serve as an alternative or adjunctive therapy to statin medications; however, the data for their use as monotherapy for reducing cardiovascular outcomes requires further study.
Commentary based on: Cheeley MK, Saseen JJ, Agarwala A, et al. NLA scientific statement on statin intolerance: a new definition and key considerations for ASCVD risk reduction in the statin intolerant patient. J Clin Lipidol 2022;June 9:[Epub ahead of print]. 1
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death globally and remains a major cause of morbidity, mortality, and disability. The role of cholesterol in the development of atherosclerosis is well established and appropriate use of LDL-C lowering therapies, particularly statins, lowers risk for ASCVD events and reduces cardiovascular mortality.2 Statins reduce ASCVD events by multiple mechanisms including plaque stabilization, reduction in inflammation, and regression of atherosclerosis.3 Despite these benefits, the use of statins and optimizing treatment dosing can be undermined in practice due to true or perceived intolerance.4
Statin intolerance has been reported in 5-30% of patients (lowest in clinical trials; highest in observational studies) and approximately 60% of adults report muscle symptoms as the primary reason for discontinuing the medication.5,6 The National Lipid Association (NLA) first proposed a working definition of statin intolerance in 2014 and their most recent 2022 statement provides an updated definition with rationale and current evidence on the prevalence and management of statin intolerance.
Statin intolerance refers to a spectrum of adverse symptoms and signs experienced by patients associated with statin therapy. The most common complaints are various muscle symptoms (soreness, aches, weakness, cramps) typically affecting symmetrical, large, and proximal muscle groups usually without creatine kinase (CK) elevation.7 Very rarely, myopathy or rhabdomyolysis may occur at a rate of 1 in 10,000 patients per year, simvastatin being the most commonly implicated statin.8,9
Other adverse signs or symptoms include liver toxicity and dysglycemia.10 The mechanism by which statins cause muscle symptoms is poorly understood but not thought to be always directly attributable to statin use. Importantly, the NLA statement reasserts that potential modifiable contributors such as vitamin D deficiency, medication interactions, excessive alcohol use, or hypothyroidism, among other entities that should also be evaluated for and corrected where possible.11
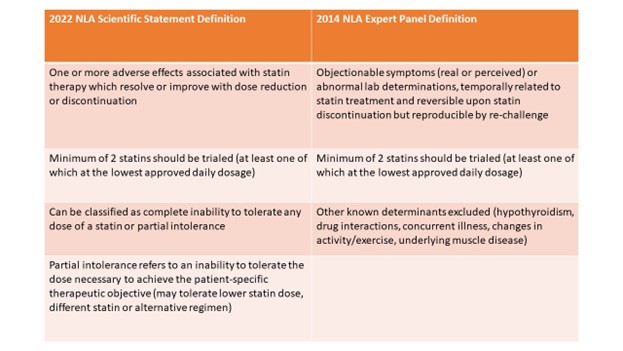
The 2022 NLA definition holds similarities as compared with the 2014 definition; a minimum of two statins should be trialed (at least one of which at the lowest approved daily dosage) (Table 1). However, the definition differs with other previously proposed definitions as it categorizes statin intolerance as either complete or partial. Partial intolerance refers to patients who can tolerate a lower statin dose, different statin, or alternative regimen, but to an extent that is insufficient to achieve the desired intensity of statin therapy or degree of LDL-C reduction.
Table 1
The incidence of true statin intolerance is difficult to ascertain and variously reported due to the challenges with diagnosis and the significant nocebo effect associated with the medication.7 Several placebo controlled have also been conducted, which demonstrate the nocebo effect more clearly. For example, in the Effects of Statins on Muscle Performance (STOMP) study randomized statin-naïve patients to atorvastatin 80 mg daily or placebo for 6 months and found that 4.6% of the placebo arm developed unexplained muscle symptoms compared with 9.4% of treatment arm.12
The ODYSSEY ALTERNATIVE trial studied statin-intolerant patients (who had attempted at least two statins, including one at the lowest dose and stopped due to muscle-related side effects by medical history) with an LDL-C ≥70 mg/dl. Patients were randomized in a 2:2:1 fashion to alirocumab 75 mg subcutaneously every 2 weeks, ezetimibe 10 mg daily, or atorvastatin 20 mg daily. It similarly demonstrated that in patients with prior self-reported statin intolerance almost 80% could subsequently tolerate low-dose atorvastatin therapy.
The Goal Achievement After Utilizing an Anti-PCSK9 Antibody in Statin Intolerant Subjects (GAUSS)-3 trial featured a crossover design in which patients with previously diagnosed statin-intolerance were randomized to atorvastatin 20 mg daily or placebo for 10 weeks separated by a 2-week washout period. While the frequency of symptoms was higher on atorvastatin, less than half of patients reported symptoms while taking atorvastatin but not while taking placebo, further substantiating that the nocebo effect contributes significantly to reported statin intolerance.13
Additionally findings from the no-of-1 studies Self-Assessment Method for Statin Side-effects or Nocebo (SAMSON) and Statin Web-based Investigation of Side Effects (StatinWISE) in which patients were provided with treatment sequences of 1-2 months of atorvastatin 20 mg or placebo, further support the view that a significant proportion of statin intolerance can be attributed to nocebo with SAMSON reporting that 90% of the symptom burden elicited by statins was also elicited by placebo.14,15 Nonetheless, it is important to note that whether causal or not, statin-associated muscle symptoms (SAMS) remain very challenging in clinical practice and impact drug adherence, which is essential for ASCVD risk reduction.
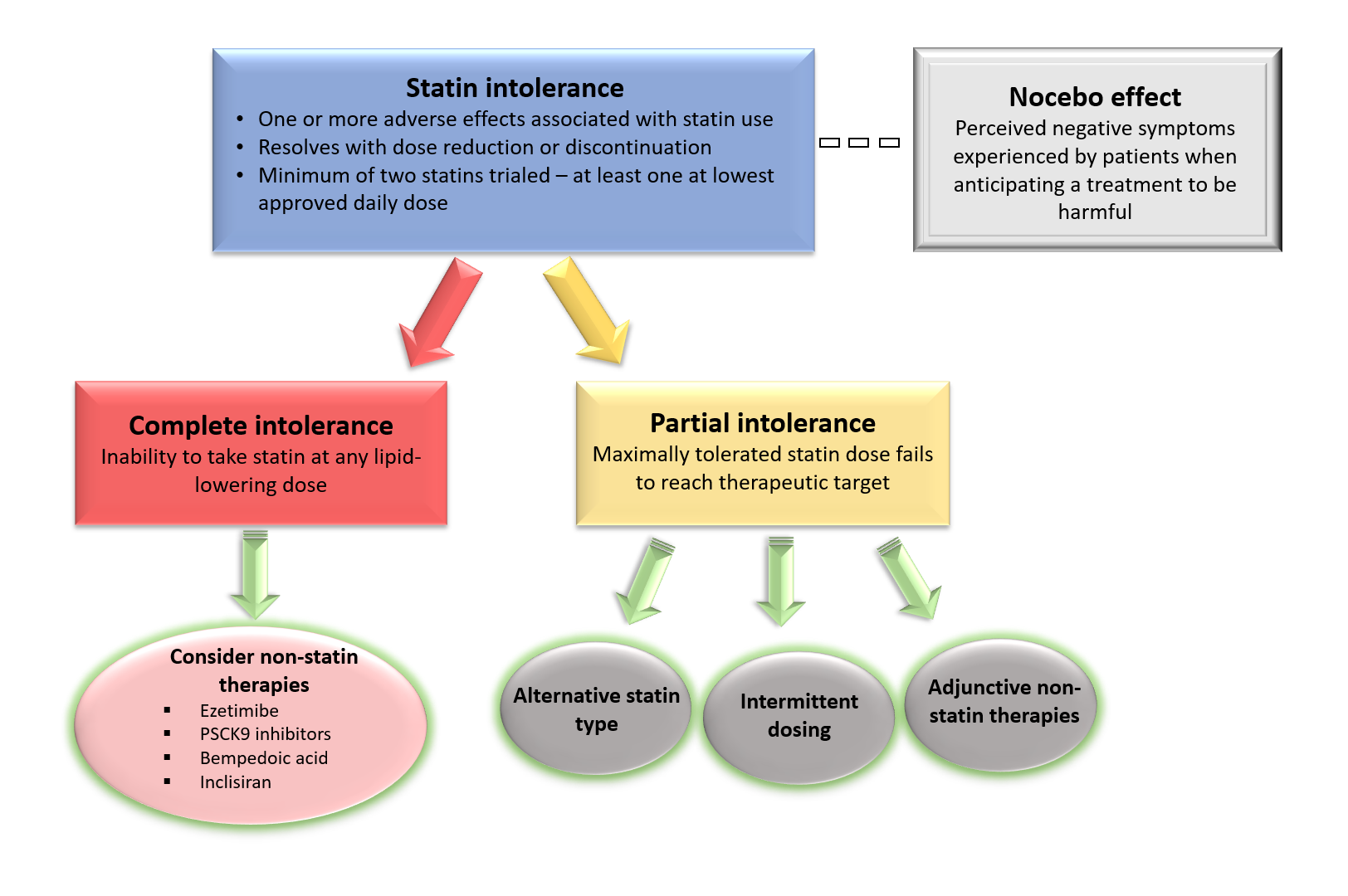
For potential solutions to statin intolerance, it is important to determine whether partial or complete statin intolerance is present. Given that complete intolerance is relatively rare (<5% of patients), an acceptable statin regimen can often be identified for most patients in the form of lower dosing, rechallenging with a different statin, or an alternative dosing schedule.16 For those with complete statin intolerance or in patients with partial intolerance who are not achieving therapeutic targets, addition of non-statin therapies which have evidence supporting reduction in ASCVD events can be considered.
Ezetimibe, which reduces LDL-C levels by approximately 20%, is the only medication at present which has shown benefit as monotherapy in reducing ASCVD event risk; however, the CLEAR-Outcomes trial is currently evaluating bempedoic acid monotherapy for ASCVD risk reduction in patients with statin intolerance.17 The monoclonal PCSK9 inhibitors produce profound LDL-C lowering effects (60% on background statin therapy) and ASCVD risk reduction in combination with maximally tolerated statin therapy.18
In summary, the updated NLA scientific statement provides a novel definition of the syndrome, separating intolerance into partial or complete as defined by ability to achieve therapeutic targets with maximally tolerated dosing. Several lipid-lowering therapies have been developed which can be considered as add-on therapy in those with partial intolerance with the goal of attaining therapeutic targets or as alternative therapy in those with complete intolerance to statins. Further research is needed to determine the benefit of novel lipid lowering therapies such as PCKS9 monoclonal antibodies or siRNA as monotherapy for this indication.
Figure 1
References
- Cheeley MK, Saseen JJ, Agarwala A, et al. NLA scientific statement on statin intolerance: a new definition and key considerations for ASCVD risk reduction in the statin intolerant patient. J Clin Lipidol 2022;Jun 9:[Epub ahead of print].
- Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol 2019;74:e177-e232.
- Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267-78.
- Bytyci I, Penson PE, Mikhailidis DP, et al. Prevalence of statin intolerance: a meta-analysis. Eur Heart J 2022;Feb 16:[Epub ahead of print].
- Jacobson TA, Cheeley MK, Jones PH, et al. The STatin Adverse Treatment Experience Survey: experience of patients reporting side effects of statin therapy. J Clin Lipidol 2019;13:415-24.
- Wei MY, Ito MK, Cohen JD, Brinton EA, Jacobson TA. Predictors of statin adherence, switching, and discontinuation in the USAGE survey: understanding the use of statins in America and gaps in patient education. J Clin Lipidol 2013;7:472-83.
- Patel J, Martin SS, Banach M. Expert opinion: the therapeutic challenges faced by statin intolerance. Expert Opin Pharmacother 2016;17:1497-507.
- Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy—European Atherosclerosis Society consensus panel statement on assessment, aetiology and management. Eur Heart J 2015;36:1012-22.
- Mendes P, Robles PG, Mathur S. Statin-induced rhabdomyolysis: a comprehensive review of case reports. Physiother Can 2014;66:124-32.
- Newman CB, Preiss D, Tobert JA, et al. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol 2019;39:e38-e81.
- Ward NC, Watts GF, Eckel RH. Statin Toxicity. Circ Res 2019;124:328-50.
- Parker BA, Capizzi JA, Grimaldi AS, et al. Effect of statins on skeletal muscle function. Circulation 2013;127:96-103.
- Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA 2016;315:1580-90.
- Howard JP, Wood FA, Finegold JA, et al. Side effect patterns in a crossover trial of statin, placebo, and no treatment. J Am Coll Cardiol 2021;78:1210-22.
- Herrett E, Williamson E, Brack K, et al. Statin treatment and muscle symptoms: series of randomised, placebo controlled n-of-1 trials. BMJ 2021;372:n135.
- Awad K, Mikhailidis DP, Toth PP, et al. Efficacy and safety of alternate-day versus daily dosing of statins: a systematic review and meta-analysis. Cardiovas Drugs Ther 2017;31:419-31.
- Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75). Circulation 2019;140:992-1003.
- Gaine SP, Quispe R, Patel J, Michos ED. New strategies for lowering low-density lipoprotein cholesterol for cardiovascular disease prevention. Curr Cardiovasc Risk Rep 2022;Jun 25:[Epub ahead of print].
Clinical Topics: Dyslipidemia, Lipid Metabolism, Nonstatins, Novel Agents, Statins
Keywords: Antibodies, Monoclonal, Atherosclerosis, Atorvastatin, Cardiovascular Diseases, Cholesterol, LDL, Cause of Death, Cross-Over Studies, Creatine Kinase, Ezetimibe, Hydroxymethylglutaryl-CoA Reductase Inhibitors, Hypothyroidism, Inflammation, Liver, Medication Adherence, Muscle Cramp, Muscles, Muscular Diseases, Nocebo Effect, Pain, PCSK9 protein, human, Prevalence, Proprotein Convertase 9, Rhabdomyolysis, Risk Reduction Behavior, RNA, Small Interfering, Self Report, Self-Assessment, Simvastatin, Vitamin D Deficiency
< Back to Listings