National Trends in Gestational Diabetes: The Importance of Data Disaggregation
Quick Takes
- Rates of gestational diabetes mellitus (GDM) increased significantly from 47.6 to 63.5 per 1,000 live births from 2011 to 2019 in a national analysis of 12 million birth records.
- Among disaggregated racial groups, Asian Indians and Puerto Rican women were found to have the highest rates of GDM.
- Future studies should explore cardiovascular disease among disaggregated racial groups to help clarify the utility of race-based, diagnostic screening thresholds.
Introduction
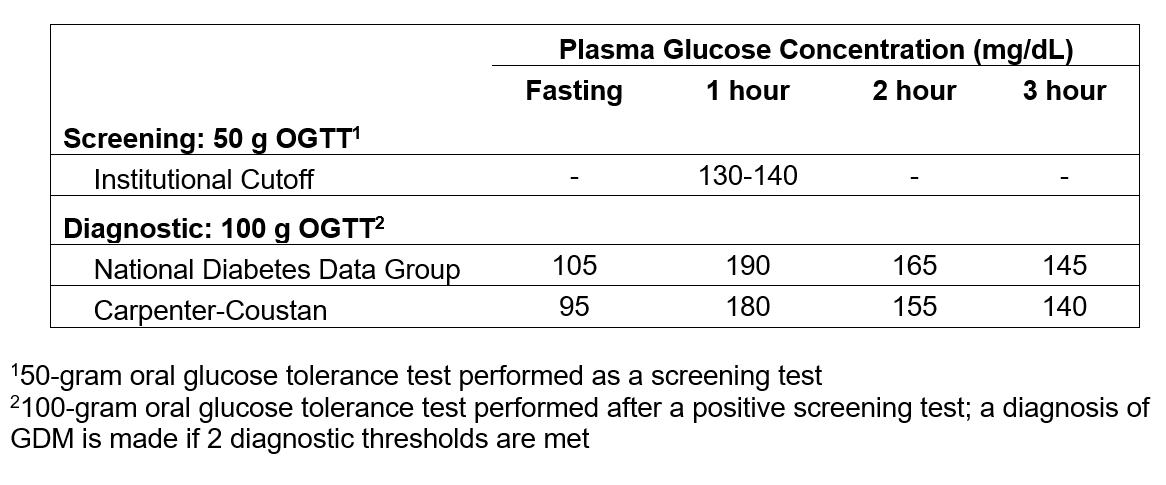
Gestational diabetes mellitus (GDM) is defined by the presence of hyperglycemia in the second or third trimester in the absence of diabetes occurring before gestation. In the United States (US), screening for GDM is recommended at 24-28 weeks of gestation and is diagnosed using a two-step approach (Table 1).1,2 However, controversy with respect to the optimum timing and diagnostic cutoffs for GDM between various professional societies persists, making the role of screening in women at increased risk for GDM less clear.3
Table 1
The diagnosis of GDM carries significant consequences for both mothers and their newborns.4,5 Offspring of women with GDM have an increased risk of macrosomia and neonatal hypoglycemia and an increased lifetime risk of developing obesity and type 2 diabetes (T2D).5 Women who develop GDM are at increased risk of developing complications during pregnancy, including hypertensive disorders of pregnancy.6 Furthermore, women with a history of GDM have an estimated two-fold increased risk of cardiovascular events in the postpartum period and as many as 70% may develop T2D later in life.7
Maternal overweight status and obesity, later age at childbearing, and family history of T2D are risk factors for GDM.8 The relationship between race and GDM has also been explored, with the highest rates occurring in Asian and Hispanic women.9 However, there is a paucity of data studying the heterogeneity of GDM risk among these racial/ethnic groups and the role of different screening strategies among these populations. Hence, the manuscript by Shah et al.10 is timely as it sought to explore changes in rates of GDM among individuals at first live birth in the US over time, and if any differences were found by race and ethnicity.
Methods and Results
Shah et al. conducted a cross-sectional analysis using birth registration records from the National Center for Health Statistics (NCHS) to examine GDM rates among pregnant women from 2011 to 2019.10 Over 12 million birth certificates were analyzed to determine age-standardized rates of GDM per 1,000 live births overall and among different race and ethnicity groups.
Rates of GDM increased significantly from 47.6 (95% confidence interval [CI], 47.1-48.0) to 63.5 (95% CI, 63.1-64.0) per 1,000 live births from 2011 to 2019, with a mean annual percent change of 3.7% (95% CI, 2.8-4.6) per year. Rates of GDM were highest among those identifying as Asia/Pacific Islander and Hispanic/Latina. When subcategories of race were analyzed, rates of GDM (per 1,000 live births) were found to be heterogenous: among those identifying as Asian or Pacific Islander, the highest rates occurred in those identifying as Asian Indian (129.1) and the lowest occurred among those identifying as Japanese (53.8). Among those identifying as Hispanic/Latina, the highest rates of GDM (per 1,000 live births) occurred in those identifying as Puerto Rican (75.8) and the lowest among those identifying as Central/South American (52.0).10
Perspective
The risk of GDM in pregnancy is increasing and is of critical importance considering the association between GDM and fetal and maternal cardiovascular outcomes. The results of the current study highlight the importance of disaggregation of racial data and underscore the importance of risk stratification and prevention, with the recognition of pregnancy and the puerperium (the so-called fourth trimester) as a window into future cardiovascular health.
There is a growing body of literature examining the mechanisms by which race/ethnicity is associated with GDM, recognizing that race is a social construct that may represent structural determinants of health. Shah et al. reported that pregestational body mass index (BMI), insurance status, and educational attainment were linked to GDM.10 They found Hispanic/Latina individuals had higher BMI and lower education than non-Hispanic White subjects, whereas Asian Indian individuals had the highest rates of GDM despite lower BMI and higher education. They hypothesized that disparities in access to prenatal care and screening for hyperglycemia may influence the risk of GDM among these populations.10
South Asians tend to have a higher percentage of body fat and more visceral adipose tissue compared to other racial groups, which may explain the higher risk of atherosclerotic cardiovascular disease (ASCVD) even at lower BMI.11 However, Asian Americans, when aggregated as a group, have been shown to be at lower risk of ASCVD, which has been attributed to the lower ASCVD event rates found in East Asians.12 When disaggregated, South Asians have higher mortality rates from ASCVD, in part due to the higher rates of metabolic syndrome, T2D, abdominal obesity, hypertension, and dyslipidemia.12,13 In the current study, Asian Indian women had the highest rate of GDM, representing a population at increased risk despite not having standard risk factors for GDM (i.e., elevated BMI).
Recent efforts have focused on the importance of disaggregation of racial/ethnic data to uncover these gaps in health outcomes.14,15 Considering discussion surrounding the optimal diagnostic cut-point for which a diagnosis of GDM should be made, the study's findings also raise the question of whether race-based cut-points or earlier screening timelines for GDM may alter ASCVD outcomes.
A recent randomized controlled trial in New Zealand comparing lower to higher glycemic criteria for the diagnosis of GDM showed no difference in the risk of the primary outcome (large-for-gestational infant) between subgroups among all races.16 Race-based screening criteria have been explored in other areas of cardiovascular disease – for instance, screening for T2D at a lower BMI in Asian Americans.17,18 Other screening strategies, such as the use of first trimester hemoglobin A1c as a predictor of GDM has a low sensitivity in predicting the development of GDM, although there are few studies exploring how different screening and diagnostic strategies may improve neonatal and maternal outcomes.19,20 The American College of Obstetrics and Gynecology currently recommends consideration of earlier screening prior to the standard second trimester screening in women who have BMI >25 or Asian Americans who have BMI >23 with other risk factors for GDM (e.g., physical inactivity, hypertension, or history of cardiovascular disease).1
In summary, the prevalence of GDM is increased across all race and ethnicities, although differences in absolute GDM rates were observed. Asian Indian and Puerto Rican women are at particular risk of adverse cardiovascular outcomes, and pregnancy can serve as a window into their future cardiovascular risk such that efforts should be made to identify and manage traditional risk factors. Future studies should explore cardiovascular outcomes and the utility of screening and diagnostic criteria among disaggregated racial groups.
References
- ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol 2018;131:e49-e64.
- Davidson KW, Barry MJ, Mangione CM, et al. Screening for gestational diabetes: US Preventive Services Task Force Recommendation statement. JAMA 2021;326:531.
- Huhn EA, Rossi SW, Hoesli I, Göbl CS. Controversies in screening and diagnostic criteria for gestational diabetes in early and late pregnancy. Front Endocrinol 2018;9:696.
- McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primer 2019;5:47.
- Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991-2002.
- Kwapong YA, Boakye E, Wang G, et al. Maternal glycemic spectrum and adverse pregnancy and perinatal outcomes in a multiracial US cohort. J Cardiovasc Dev Dis 2022;9:179.
- Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373:1773-79.
- Egan AM, Dow ML, Vella A. A Review of the Pathophysiology and Management of Diabetes in Pregnancy. Mayo Clin Proc. 2020;95:2734-46.
- Perera MJ, Reina SA, Elfassy T, et al. Gestational diabetes and cardiovascular risk factors and disease in U.S. Hispanics/Latinas in the Hispanic Community Health Study/Study of Latinos (HCHS/SOL). Women Health 2019;59:481-95.
- Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA 2022;42:64-65.
- Gupta M, Brister S, Verma S. Is South Asian ethnicity an independent cardiovascular risk factor? Can J Cardiol 2006;22:193-97.
- Volgman AS, Palaniappan LS, Aggarwal NT, et al. Atherosclerotic cardiovascular disease in South Asians in the United States: epidemiology, risk factors, and treatments: a scientific statement from the American Heart Association. Circulation 2018;138:e1-e34.
- Misra A, Shrivastava U. Obesity and Dyslipidemia in South Asians. Nutrients 2013;5:2708-33.
- Rodriguez F, Chung S, Blum MR, Coulet A, Basu S, Palaniappan LP. Atherosclerotic cardiovascular disease risk prediction in disaggregated Asian and Hispanic subgroups using electronic health records. J Am Heart Assoc 2019;8:e011874.
- Findlay SG, Kasliwal RR, Bansal M, Tarique A, Zaman A. A comparison of cardiovascular risk scores in native and migrant South Asian populations. SSM - Popul Health 2020;11:100594.
- Crowther CA, Samuel D, McCowan LME, Edlin R, Tran T, McKinlay CJ. Lower versus higher glycemic criteria for diagnosis of gestational diabetes. N Engl J Med 2022;387:587-98.
- Caleyachetty R, Barber TM, Mohammed NI, et al. Ethnicity-specific BMI cutoffs for obesity based on type 2 diabetes risk in England: a population-based cohort study. Lancet Diabetes Endocrinol 2021;9:419-26.
- Hsu WC, Araneta MRG, Kanaya AM, Chiang JL, Fujimoto W. BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes Care 2015;38:150-58.
- Raets L, Beunen K, Benhalima K. Screening for gestational diabetes mellitus in early pregnancy: what is the evidence? J Clin Med 2021;10:1257.
- Osmundson S, Zhao B, Kunz L, et al. First trimester hemoglobin A1c prediction of gestational diabetes. Am J Perinatol 2016;33:977-82.
Clinical Topics: Cardiovascular Care Team, Diabetes and Cardiometabolic Disease, Dyslipidemia, Prevention, Vascular Medicine, Hypertension
Keywords: Diabetes, Gestational, Pregnancy, Ethnic Groups, Body Mass Index, Cross-Sectional Studies, Cardiovascular Diseases, Glycated Hemoglobin A, Asian Americans, Prevalence, Prenatal Care, Diabetes Mellitus, Type 2, Confidence Intervals, Gynecology, Hypertension, Pregnancy-Induced, Intra-Abdominal Fat, Metabolic Syndrome, National Center for Health Statistics, U.S., Obesity, Abdominal, Obstetrics, Overweight, Pregnancy Trimester, First, Pregnancy Trimester, Second, Pregnancy Trimester, Third, Pregnant Women, Pregnant Women, Sedentary Behavior, Social Determinants of Health, Risk Factors, Heart Disease Risk Factors, Cardiometabolic Risk Factors, Hispanic Americans, Postpartum Period, Risk Assessment, Insurance Coverage, Outcome Assessment, Health Care, Hyperglycemia, Dyslipidemias, Hypoglycemia
< Back to Listings