Emerging Frontier in Heart Transplantation: Donation After Circulatory Death
Quick Takes
- Machine perfusion enabled the success of DCD heart transplant by mitigating previous deleterious effects of warm ischemia.
- DCD and DBD heart transplant have similar outcomes.
- Widespread adoption of DCD heart transplant can expand the donor pool, resulting in increased transplant volume and decreased waitlist times.
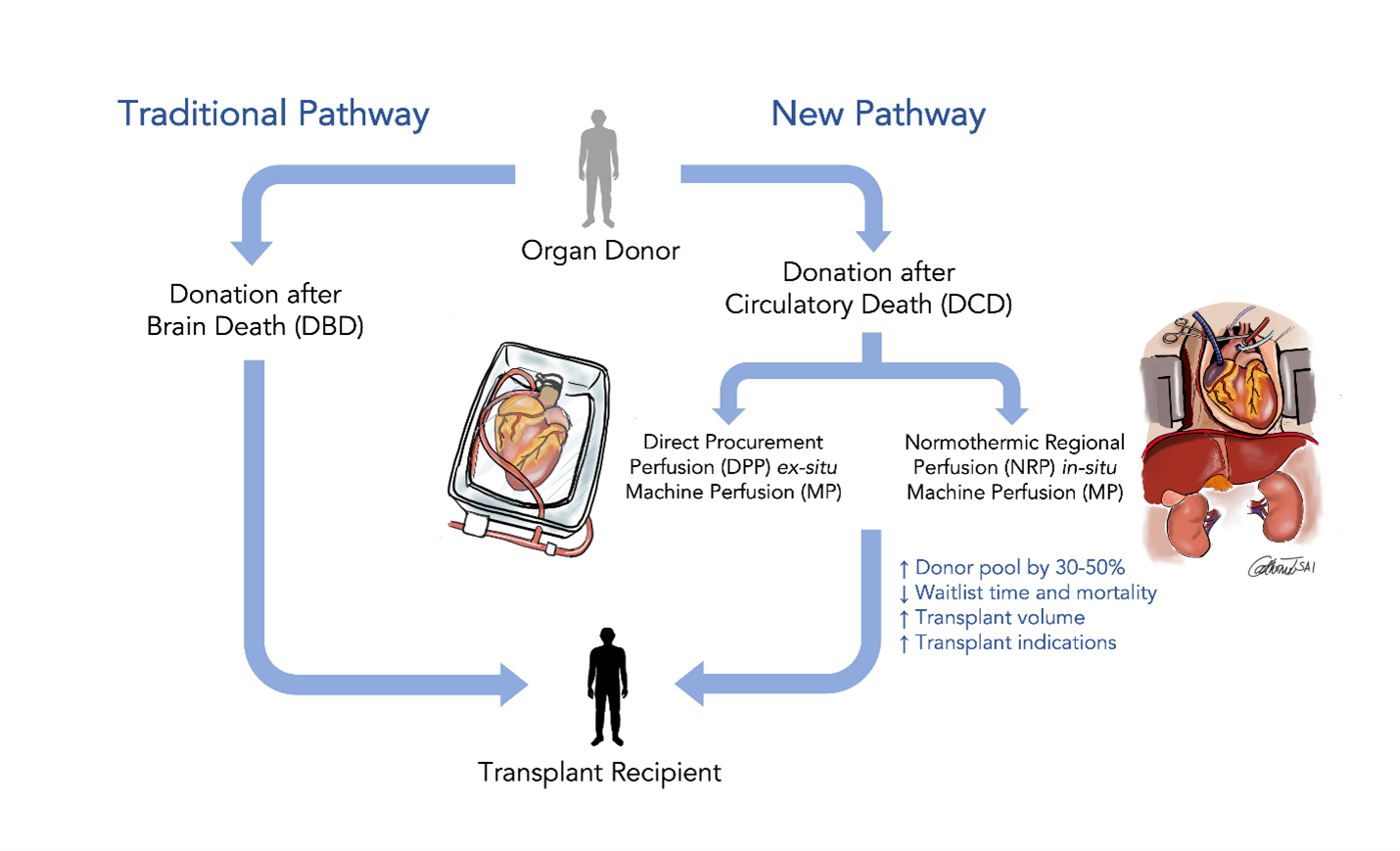
Figure 1
Background
Organ availability is the main factor limiting access to heart transplantation. Therefore, transplant programs use marginal donors with risk factors including older age, size mismatch, left ventricular hypertrophy, low ejection fraction, and longer donor ischemic times.1
Deceased donor organ donation proceeds after declaration of brain death (DBD) or circulatory death (DCD). Brain death is declared when all brain functions have ceased. Scheduled organ evaluation and procurement in DBD donors is a well-established process, whereby organ function is arrested with cold solution flushing. Circulatory death donors do not meet full brain death criteria, but have suffered devastating, irreversible injuries and further care is futile. Following withdrawal of care, the donor expires, and death is declared on circulatory basis (no blood pressure, pulse, or heartbeat). Following a standoff period of typically 5 minutes, circulatory death is reconfirmed prior to proceeding with organ procurement.
Ischemic injury is a major risk to all donor organs. DCD hearts experience severe ischemic injury, to the point of loss of pump and electrical function. Recent improvements in machine perfusion (MP) have allowed resuscitation of DCD hearts and successful transplant. Estimates suggest that DCD hearts can increase the donor pool by as much as 50%.2-4
Method of DCD Heart Recovery
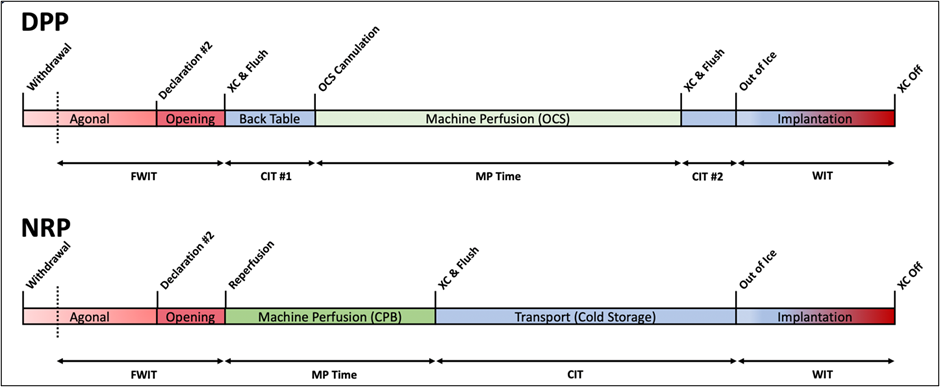
Two techniques for DCD heart procurement have emerged: direct procurement and ex situ perfusion (DPP) and normothermic regional in situ perfusion (NRP).
The DPP method relies on ex situ MP. After confirmation of circulatory death, the heart is flushed with cold crystalloid cardioplegia and procured, followed by back table instrumentation and reperfusion with normothermic blood. TransMedics Organ Care System (OCS™) (Andover, MA, USA) is the only FDA-approved system for ex situ perfusion of DCD hearts. This method requires harvesting 1100 mL donor blood for OCS machine priming prior to heart recovery. The aorta and pulmonary artery are cannulated, and a left ventricular vent prevents distension. The heart is connected to the OCS system via the aorta and kept in a normothermic, beating condition during resuscitation and transport. Lactate metabolism, visual inspection and coronary flow are used as surrogates for cardiac function and organ viability. The heart is cooled and arrested with cardioplegia before implant.
The NRP method relies on an open cardiopulmonary bypass (CPB) circuit. Standard DCD procedure for declaration of death and standoff period are followed. Thereafter, the donor right atrium and ascending aorta are cannulated for CPB. Next, the aortic arch vessels are clamped to exclude brain circulation from the rest of the body before initiating thoraco-abdominal organ reperfusion with CPB. Lack of perfusion to the brain is further ensured by venting the innominate artery. Typically, within 45 – 60 minutes, heart function has recovered. CPB is weaned, allowing formal assessment of heart function. The heart is then arrested with cardioplegia and transported using either cold static storage or normothermic or hypothermic MP storage.
Figure 2: Methods of Donation after Circulation Death
DPP = direct procurement and machine perfusion, NRP = normothermic regional perfusion, f-WIT = functional warm ischemic time, XC = aortic cross clamp, MP = machine perfusion, CIT = cold ischemic time, WIT = warm ischemic time
Evolution of DCD Heart Transplantation
Use of DCD hearts is not a novel concept. The first heart transplant was performed in 1967 by Dr. Christiaan Barnard with a DCD donor using NRP. However, with the development of brain death criteria and concern for ischemic cardiac injury, the field moved away from DCD. It was not until 2004 when Denver Children's Hospital performed three successful pediatric DCD heart transplants.5 Ten years later, a DCD program was established in St. Vincent's Hospital in Sydney, Australia utilizing DPP.6 In 2015, Papworth Hospital in Cambridge, UK performed DCD heart transplants using NRP.7
In the United States (US), the first adult DCD heart transplant was performed in 2019 as part of a randomized controlled trial comparing DCD heart transplant using the OCS versus DBD using cold storage.8 In 2020, New York University (NYU) performed the first adult DCD NRP heart transplant in the US.9
Establishing a DCD heart transplant program has been shown to increase overall organ yield per donor.10 Thoraco-abdominal NRP has demonstrated improved DCD liver and kidney graft quality and survival.11
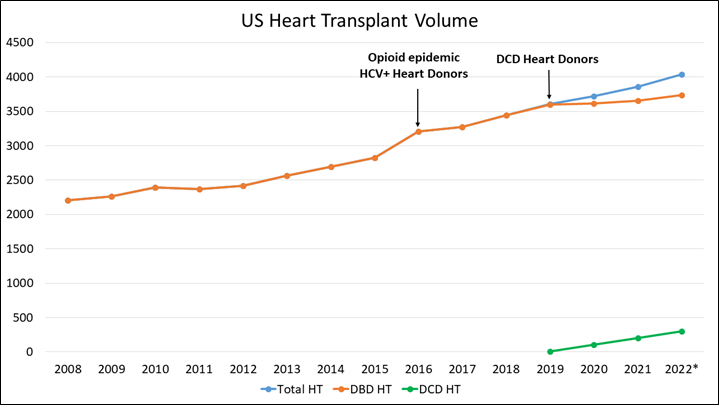
Figure 3: US Heart Transplant Volume
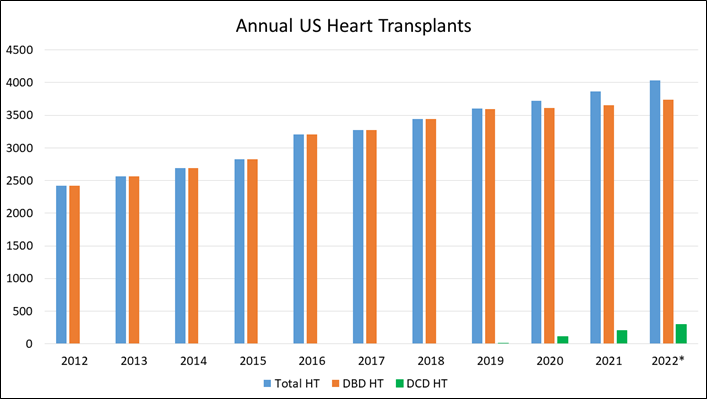
Figure 4: Annual US Heart Transplants
*2022 transplant numbers are annualized based on the number of transplants YTD August 31, 2022. Courtesy of Lee C, Tsai C, Adler E, Pretorius V.
Outcomes
International data show a 30-48% increase in donor pool with similar outcomes to DBD heart transplant.3
Messer and colleagues compared 79 DCD to 79 DBD heart transplants. They found comparable 90-day (DCD 92% vs. DBD 96%) and 1-year survival (DCD 91% vs. DBD 89%). There was no significant difference in primary graft dysfunction requiring extracorporeal membrane oxygenation (ECMO) (DCD 15% vs. DBD 6%, p = 0.12) or intra-aortic balloon pump (IABP) (DCD 32% vs. DBD 23%, p=0.28). There was also no significant difference in intensive care unit (ICU) or hospital lengths of stay. Of the DCD transplants, 57 hearts were DPP procured and 22 NRP followed by OCS perfusion. Comparing these two methods, survival was similar, but NRP transplants required less ventilator days, lower need for dialysis, and shorter hospital stay.3
US DCD heart multicenter trial randomized 180 patients — 90 OCS DCD hearts and 90 DBD hearts. Six- and 12-month post-transplant survival were comparable. Utilization rate for DCD hearts procured was 89%.8
Table 1: Published Outcomes on DCD Heart Transplant as of 10/2022
| Papworth UK | St. Vincent's Australia |
London UK1 | US2 | NYU | Vanderbilt3 | UCSD4 | |
| n | 79 | 49 | 36 | 127 | 8 | 15 | 74 |
| DCD Method | DPP, NRP + OCS | DPP | DPP | DPP, NRP + CSS | NRP + CSS | NRP + CSS | DPP, NRP + CSS |
| Acceptance rate | 32 (65%) | 25 (69%) | 100% | * | 89% | ||
| Severe PGD5 | 15% | 10 (31%) | 20 (80%) | 1 (12.5%) | 0 | 6 (8.1%) | |
| 30-day survival | 97% | 88% | 96.8% | 100% | 100% | 98.6% | |
| 1-yr survival | 91% | 96% | 92.5% | 98.6% |
Ethical Considerations
While DCD organ donation is well established, ethical concerns arise with conduct of DCD heart transplant. Determination of death by circulatory criteria is defined by irreversible cessation of cardiopulmonary function. The term "irreversible" raises concerns regarding violating the dead donor rule by restoring circulation to the thoraco-abdominal organs with NRP.14 However, the donor has already met criteria and been declared deceased prior to any intervention. The mandatory standoff time confirms absence of autoresuscitation. And restoring circulation to the thoraco-abdominal organs with NRP does not in affect resuscitate the person or reverse already non-life sustaining damage that occurred prior to withdrawal.15-18 To further address any concerns regarding potential for resumption of neurologic function with NRP, the head vessels are occluded prior to starting bypass and vented.19
It is important to note that if the time from withdrawal of life support to death exceeds the acceptable f-WIT for solid organ donation, donors remain on hospice and the progression to death continues. In this instance, the donor may still be eligible to donate other tissue for transplant or research.
Conclusion
Widespread adoption of DCD heart transplant had been limited by ischemic injury. However, success of using DPP and NRP techniques has resulted in exponential growth of DCD heart transplant in recent years. Furthermore, MP provides a platform to investigate novel therapeutics, such as gene therapy or mitochondrial transplant, potentially one day allowing repair and reimplant of a failing heart.
Many benefits of DCD heart transplant support its wider spread adoption: expanding the donor pool, decreasing waitlist times, improving organ quality, increasing total organ yield per donor are among these.
References
- Dharmavaram N, Hess T, Jaeger H, et al. National trends in heart donor usage rates: are we efficiently transplanting more hearts? J Am Heart Assoc 2021;10:e019655.
- Madan S, Saeed O, Forest SJ, Goldstein DJ, Jorde UP, Patel SR. Feasibility and potential impact of heart transplantation from adult donors after circulatory death. J Am Coll Cardiol 2022;79:148-162.
- Messer S, Cernic S, Page A, et al. A 5-year single-center early experience of heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant 2020;39:1463-75.
- Jawitz OK, Raman V, De Vore AD, et al. Increasing the United States heart transplant donor pool with donation after circulatory death. J Thorac Cardiovasc Surg 2020;159:e307-e309.
- Boucek MM, Mashburn C, Dunn SM, et al. Pediatric heart transplantation after declaration of cardiocirculatory death. N Engl J Med 2008;359:709-14.
- Dhital K, Ludhani P, Scheuer S, Connellan M, Macdonald P. DCD donations and outcomes of heart transplantation: the Australian experience. Indian J Thorac Cardiovasc Surg 2020;36:224-S32.
- Messer S, Page A, Axell R, et al. Outcome after heart transplantation from donation after circulatory-determined death donors. J Heart Lung Transplant 2017;36:1311-18.
- Schroder JN, Shah A, Pretorius V, et al. Expanding heart transplants from donors after circulatory death (DCD) – results of the first randomized controlled trial using the organ care system (OCSTM) Heart – (OCS DCD Heart Trial)J Heart Lung Transplant 2022;41:S72.
- Smith DE, Kon ZN, Carillo JA, et al. Early experience with donation after circulatory death heart transplantation using normothermic regional perfusion in the United States. J Thorac Cardiovasc Surg 2022;164:557-568.e1.
- Gauntt K, Carrico B, Klassen D. DCD heart donation: Impact on organ yield (atcmeeetingabstracts.com). 2021. Available at: https://atcmeetingabstracts.com/abstract/dcd-heart-donation-impact-on-organ-yield/. Accessed 08/07/2021.
- Hessheimer AJ, Coll E, Torres F, et al. Normothermic regional perfusion vs. super-rapid recovery in controlled donation after circulatory death liver transplantation. J Hepatol 2019;70:658-65.
- Mohite PN, Umakumar K, Garcia-Saez, et al. Heart transplantation utilizing organs following donation after circulatory death (DCD). J Heart Lung Transplant 2022;41:S175-176.
- Hoffman JRH, McMaster MG, Rali AS, et al. Early US experience with cardiac donation after circulatory death (DCD) using normothermic regional perfusion. J Heart Lung Transplant 2021;40:1408-18.
- American College of Physicians. Ethics, determination of death, and organ transplantation in normothermic regional perfusion (NRP) with controlled donation after circulatory determination of death (cDCD): American College of Physicians Statement of Concern (acponline.org). 2021. Available at: https://www.acponline.org/acp_policy/policies/ethics_determination_of_death_and_organ_transplantation_in_nrp_2021.pdf. Accessed 02/06/2022.
- Parent B, Moazami N, Wall S, et al. Ethical and logistical concerns for establishing NRP-cDCD heart transplantation in the United States. Am J Transplant 2020;20:1508-12.
- Parent B, Caplan A, Moazami N, Montgomery RA. Response to American College of Physician's statement on the ethics of transplant after normothermic regional perfusion. Am J Transplant 2022;22:1307-10.
- Wall AE, Fiedler A, Karp S, Shah A, Testa G. Applying the ethical framework for donation after circulatory death to thoracic normothermic regional perfusion procedures. Am J Transplant 2022;22:1311-15.
- Holm AM, Courtwright A, Olland A, Zuckermann A, Van Raemdonck D. ISHLT position paper on thoracic organ transplantation in controlled donation after circulatory determination of death (cDCD). J Heart Lung Transplant 2022;41:671-77.
- Manara A, Shemie SD, Large S, et al. Maintaining the permanence principle for death during in situ normothermic regional perfusion for donation after circulatory death organ recovery: a United Kingdom and Canadian proposal. Am J Transplant 2020;20:2017-25.
Clinical Topics: Cardiac Surgery, Invasive Cardiovascular Angiography and Intervention, Vascular Medicine, Aortic Surgery, Cardiac Surgery and Heart Failure, Heart Transplant, Interventions and Vascular Medicine, Heart Failure and Cardiomyopathies
Keywords: Aorta, Thoracic, Brachiocephalic Trunk, Brain, Brain Death, Death, Cardiopulmonary Bypass, Crystalloid Solutions, Extracorporeal Membrane Oxygenation, Genetic Therapy, Heart Arrest, Induced, Heart Atria, Heart Rate, Heart Transplantation, Hospices, Hospitals, Hypertrophy, Left Ventricular, Intensive Care Units, Kidney, Lactates, Liver, Length of Stay, Perfusion, Primary Graft Dysfunction, Pulmonary Artery, Reperfusion, Return of Spontaneous Circulation, Tissue and Organ Procurement, Stroke Volume, Risk Factors, Tissue Donors, Tissue Survival, Universities, Ventilators, Mechanical
< Back to Listings