Renal Denervation in the Treatment of Resistant Hypertension
Quick Takes
- Renal denervation (RDN) is a catheter-based intervention that targets and ablates sympathetic renal nerves to treat hypertension.
- Radiofrequency RDN and ultrasound RDN are the two best studied modalities currently in use in the United States.
- Multiple sham-controlled trials have demonstrated the safety, efficacy, and durability of RDN in the treatment of hypertension for patients both on and off medical antihypertensive therapy.
Hypertension (HTN) affects approximately 50% of the adult population and is one of the most important contributors to cardiovascular morbidity and mortality.1,2 Blood pressure (BP) remains sub-optimally controlled in up to 25% of these patients, often attributed to medical non-adherence and resistant disease despite appropriate medical therapy.1,3 Renal denervation (RDN) therapy is an invasive treatment modality for resistant HTN that employs catheter-based techniques to ablate the sympathetic nervous fibers coursing along the renal arteries implicated in driving hypertension.4
The two best studied platforms for RDN include radiofrequency RDN (rRDN) and ultrasound RDN (uRDN). In both systems, RDN is performed via the common femoral artery and after selective renal angiography, and placement of a 0.014" guidewire in the renal arteries. rRDN is currently performed with the Symplicity Spyral™ RDN system (Medtronic Inc.), which emits radiofrequency waves from four radiopaque electrodes located on the Spyral™ catheter's helical shaped tip (Figure 1).
Figure 1
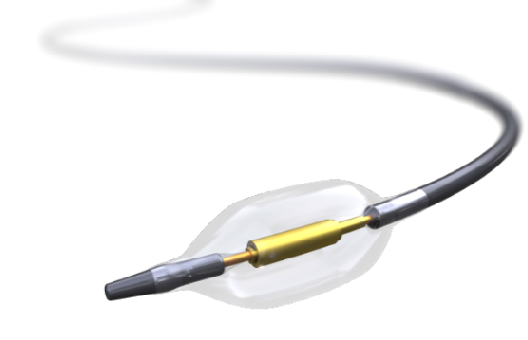
uRDN is performed with the Paradise Renal Denervation System (ReCor Medical), which contains a balloon at its distal tip surrounding an ultrasound emitting core (Figure 2). The Paradise catheter is an over-the-wire system that is attached to a console that inflates the balloon and continuously infuses sterile water to cool the arterial wall before, during, and after treatment. uRDN treats the main renal arteries, as data suggest similar efficacy between rRDN treatment of the main and distal arteries versus uRDN in the main renal artery.5
Figure 2
Contemporary data for RDN are available from two populations of patients with HTN: those not taking antihypertensive medications, and those taking antihypertensive medications. The rationale for these designs is to test efficacy of RDN in isolation in the former and to understand RDN's role as an adjunctive therapy in the latter.
In the off-medication cohort, rRDN was studied in the SPYRAL HTN-OFF MED (SPYRAL Pivotal) trial. This was a multi-center, prospective, single-blinded, sham-controlled trial that randomized 331 patients with an office systolic BP (SBP) between 150-180 mmHg and a 24-hour mean SBP 140-170 mmHg off of medications to rRDN or sham.6 The primary endpoint of the study was change in the mean 24-hour SBP from baseline to 3 months. The short duration of this endpoint was intended to limit the amount of time that patients in the sham arm were off of antihypertensive therapy. Patients randomized to rRDN demonstrated a mean reduction in SBP and diastolic BP (DBP) of 4.7 mmHg and 3.7 mmHg respectively, compared to 0.6 mmHg and 0.8 mmHg in the sham group (p <0.001 for both comparisons). The uRDN equivalent trial, RADIANCE HTN SOLO, was a multi-center, randomized, sham-controlled trial that randomized 146 patients with ambulatory SBP between 135-170 mmHg off of medication to uRDN or sham.7 Patients in the uRDN group had greater reduction in the primary endpoint compared to sham, which was daytime ambulatory SBP at 2 months (8.5 mmHg vs. 2.2 mmHg, p = 0.0001). Three-year follow up analysis of the 51 patients who underwent uRDN treatment had a lasting reduction in office BP of 18 mmHg from baseline to 36 months.8 These studies definitively prove the efficacy of RDN in isolation.
Several studies have now been published to assess the potency of RDN in the background of anti-hypertension medication. SPYRAL HTN-ON MED studied rRDN in a multi-center, randomized, sham-controlled trial that assigned 80 patients to receive rRDN or sham procedure.9 Patients randomized to rRDN had a reduction in mean 24-hour SBP of 9.3 mmHg, compared to 1.6 mmHg in the sham group (p = 0.004). This effect proved durable as well, with a reduction at 36 months of 18.7 mmHg in the RDN group compared to 8.6 mmHg in the sham group. The RADIANCE HTN TRIO trial studied uRDN in 136 patients on three or more anti-HTN medications.10 Those in the uRDN group demonstrated a reduction in daytime ambulatory SBP of 8.0 mmHg, compared to 3.0 mmHg in the sham group (p = 0.022). Both RDN studies reported minimal safety events.9,10 These studies prove that RDN has an additive role in patients with uncontrolled hypertension despite multiple anti-hypertensive medications.
Additional data on the efficacy of RDN were presented as late breaking clinical trials presented at the 2022 Transcatheter Therapeutics (TCT) meeting with the release of the RADIANCE II Pivotal trial and 3-year follow up data from SYMPLICITY HTN-3.11,12 The RADIANCE II Pivotal trial randomized 224 patients with daytime ambulatory SBP ranging from 135-170 mmHg who were on two anti-hypertensive medications in a 2:1 fashion to uRDN or sham. At 2 months, mean daytime ambulatory SBP was reduced by 7.9 mmHg in the uRDN group compared to 1.8 mmHg in the sham group (p<0.01), further demonstrating added efficacy of uRDN in a cohort of patients with a lower burden of anti-hypertensive medications.11 While the primary end-points for SYMPLICITY-HTN 3 trial were originally negative at 6 months, the 3-year follow up data, which studied the previous generation of rRDN technology, demonstrated those in the rRDN group had a sustained reduction in office SBP of 26.4 mmHg compared to only 5.7 mmHg in the sham group at 36 months.12,13 These data are in line with 3-year outcomes data from the Global Symplicity Registry, in which 1742 patients treated with rRDN showed a 16.5 mmHg reduction in office SBP compared with placebo (p <0.001).14 Further, there were no long term safety events noted in the treatment group with only small reduction seen in estimated glomerular filtration rate at 36 months.14
The safety profile for RDN has been excellent. In a recent meta-analysis including 5,769 patients in 50 RDN studies including studies utilizing first generation RDN catheters, there was only a 0.45% rate of renal artery stenosis or dissection reported.15
Taken together, multiple sham-controlled trials have demonstrated RDN to be efficacious and safe for the treatment of HTN both in isolation and in conjunction with antihypertensive medical therapy. RDN should be considered part of the treatment armamentarium for HTN when approved, and efforts should focus on the strategic, systematic, and equitable access to this paradigm shifting technology, as well as on determining which patients are most likely to respond to RDN.
References
- Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke statistics—2020 Update: a report from the American Heart Association. Circulation 2020;141:e139-e596.
- Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol 2020;16:223-37.
- Berra E, Azizi M, Capron A, et al. Evaluation of adherence should become an integral part of assessment of patients with apparently treatment-resistant hypertension. Hypertension 2016;68:297-306.
- Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med 2009;361:932-34.
- Fengler K, Rommel KP, Blazek S, et al. A three-arm randomized trial of different renal denervation devices and techniques in patients with resistant hypertension (RADIOSOUND-HTN). Circulation 2019;139:590-600.
- Böhm M, Kario K, Kandzari DE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet 2020;395:1444-51.
- Azizi M, Schmieder RE, Mahfoud F, et al. Endovascular ultrasound renal denervation to treat HTN (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 2018;391:2335-45.
- Rader F, Kirtane AJ, Wang Y, et al. Durability of blood pressure reduction after ultrasound renal denervation: three-year follow-up of the treatment arm of the randomised RADIANCE-HTN SOLO trial. EuroIntervention 2022;18:e677-e685.
- Mahfoud F, Kandzari DE, Kario K, et al. Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomised, sham-controlled trial. Lancet 2022;399:1401-10.
- Azizi M, Sanghvi K, Saxena M, et al. Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet 2021;397:2476-86.
- Endovascular ultrasound renal denervation to treat uncontrolled hypertension: primary results of the randomized, sham-controlled RADIANCE II pivotal trial. Presented by Dr. Ajay Kirtane at Transcatheter Cardiovascular Therapeutics Conference (TCT 2022), September 18, 2022.
- Bhatt DL, Vaduganathan M, Kandzari DE, et al. Long-term outcomes after catheter-based renal artery denervation for resistant hypertension: final follow-up of the randomized SYMPLICITY HTN-3 Trial. Lancet 2022;400:1405-16.
- Bhatt DL, Kandzari DE, O'Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014;370:1393-1401.
- Mahfoud F, Böhm M, Schmieder R, et al. Effects of renal denervation on kidney function and long-term outcomes: 3-year follow-up from the Global SYMPLICITY Registry. Eur Heart J 2019;40:3474-82.
- Townsend R, Walton A, Hettrick DA, et al. Review and meta-analysis of renal artery damage following percutaneous renal denervation with radiofrequency renal artery ablation. Eurointervention 2020;16:89-96.
Clinical Topics: Diabetes and Cardiometabolic Disease, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Prevention, Pulmonary Hypertension and Venous Thromboembolism, Vascular Medicine, Pulmonary Hypertension, Interventions and Imaging, Interventions and Vascular Medicine, Angiography, Nuclear Imaging, Hypertension
Keywords: Antihypertensive Agents, Blood Pressure, Femoral Artery, Renal Artery, Denervation, Cardiovascular Diseases, Angiography, Coronary Angiography, Hypertension, Hypertension, Pulmonary, Electrodes, Catheters, Follow-Up Studies, Prospective Studies, Glomerular Filtration Rate, Renal Artery Obstruction, Registries, Classification
< Back to Listings