The Paradigm Shift in the Management of Recurrent Pericarditis
Quick Takes
- Up to 30% of the patients with acute pericarditis can have recurrent pericarditis, which can be remarkably difficult to treat, with treatment duration lasting up to 5-7 years in patients with multiple recurrences.
- Since the last published guidelines in 2015, there has been tremendous growth in the data supporting the use of advanced imaging and biologics for the management of recurrent pericarditis.
- Cardiac magnetic resonance imaging and interleukin-1 inhibitors form the backbone for individualized treatment of recurrent pericarditis which has led to a paradigm shift by considerably decreasing the total treatment duration.
Introduction
Pericarditis is the most common pericardial disease. It leads to 5.4-26 hospitalizations/100,000 persons per year in the United States (US).1 The spectrum of pericarditis consists of acute, incessant, chronic, recurrent, and constrictive pericarditis. Recurrent pericarditis affects 15-30% of patients with acute pericarditis; 50% of these patients suffer from multiple recurrences.2 Management of this chronic debilitating condition involves a combination of anti-inflammatory therapies, with treatment lasting up to 4.7-6.2 years in difficult-to-treat patients.3 This article summarizes the established and emerging diagnostic and treatment techniques for patients with recurrent pericarditis. We especially emphasize what is new since the 2015 European Society of Cardiology (ESC) guidelines on pericardial diseases.
Diagnosis
ESC guidelines require two of the following four criteria to diagnose acute pericarditis: (1) chest pain consistent with pericarditis, (2) pericardial friction rub, (3) electrocardiogram (ECG) showing diffuse ST-segment elevation or PR-segment depression, and (4) new or worsening pericardial effusion.4 Recurrent pericarditis is diagnosed when a patient meets all three criteria: (1) documented episode of acute pericarditis, (2) symptom-free interval of 4-6 weeks, and (3) recurrence based on criteria described for acute pericarditis.4 However, patients with recurrent pericarditis having a recurrence of chest pain often do not meet other listed criteria.5 In such cases, diagnosis is supported by elevated inflammatory markers such as erythrocytic sedimentation rate (ESR) or C-reactive protein (CRP), or by imaging studies such as cardiac magnetic resonance imaging (CMR) or cardiac computed tomography (CT) showing signs of pericardial inflammation.
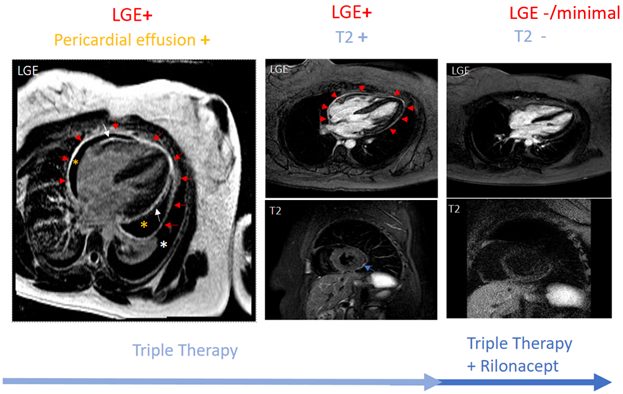
In the absence of disease-specific inflammatory markers, data have rapidly evolved supporting the use of imaging in pericarditis. Guidelines recommend echocardiography as the first imaging test for all pericardial diseases.4,6 Other imaging modalities such as CMR have traditionally been reserved for unclear cases. However, lately, CMR sequencing has been used to provide pericardial characterization by detailing the anatomy and quantifying pericardial inflammation. The presence of T2 and late gadolinium enhancement (LGE) has a sensitivity of 73% and specificity of 99% for diagnosing recurrent pericarditis.7 The edema-weighted T2 short-tau inversion recovery sequence and the LGE sequence of CMR play a key role in providing pericardial characterization. This characterization informs as to where the patient stands along the spectrum of pericarditis (acute vs. recurrent vs. burnt-out). An increased pericardial signal on T2 signifies edema, while an increased pericardial signal on LGE suggests underlying inflammation.8,9 An increased pericardial signal on LGE as well as T2 (i.e., LGE+ and T2+) is consistent with new acute inflammation or recurrence of inflammation. LGE+ with T2- suggests subacute/chronic inflammation. An absence of pericardial signal on LGE and T2 (i.e., LGE- and T2-) is consistent with the absence of active inflammation. Figure 1 shows CMR findings at various stages of pericardial inflammation as noted in a patient receiving treatment for recurrent pericarditis. CMR can also assist in prognosticating by identifying patients at higher risk of complications such as recurrence, constriction, or tamponade.7,10
Figure 1
Management
Table 1 summarizes the randomized controlled trials supporting the use of medical therapies for pericarditis.
Table 1: Randomized Controlled Trials of Therapies for Pericarditis
| Drugs | Trial, year | Study population | Primary endpoint | Results |
| Colchicine | CORE 2005 |
First recurrence of pericarditis | Recurrence rate | 24% in colchicine + aspirin group and 50.6% in aspirin only group (NNT: 4 [95% CI, 2.5-7.1]; p: 0.02) |
| COPE 2005 |
Acute pericarditis | Recurrence rate | 10.7% in colchicine + aspirin group and 32.3% in aspirin only group (NNT: 5; p: 0.004) | |
| CORP 2011 |
First recurrence of pericarditis | Recurrence rate | 24% in colchicine + NSAIDs group and 55 in NSAIDs + placebo group (RRR: 0.56 [95% CI 0.27-0.73]; NNT: 3; p<0.001) | |
| ICAP 2013 |
Acute pericarditis | Incessant/recurrent pericarditis | 16.7% in colchicine + NSAIDs group and 37.5% in NSAIDs + placebo group (RRR: 0.56 [95% CI 0.30-0.72]; NNT: 4; p<0.001) | |
| CORP-2 2014 |
≥2 recurrences of pericarditis | Recurrence of pericarditis | 21.6% in colchicine + NSAIDs group and 42.5% in placebo + NSAIDs group (RR: 0.49 [95% CI 0.24-0.65]; NNT: 5; p<0.001) | |
| Anakinra | AIRTRIP 2016 |
Corticosteroid dependent colchicine resistant recurrent pericarditis with ≥3 recurrences and elevated CRP | Recurrence of pericarditis and time to recurrence | Recurrence: 2/11 in anakinra group and 9/10 in placebo group Median time to recurrence: median time was 72 days (64-150) in placebo group and was not reached in anakinra group (p<0.001) |
| Rilonacept | RHAPSODY 2021 | Recurrent pericarditis with ≥ recurrences, CRP>1 mg/dL, and pain rating ≥4 despite NSAIDs, colchicine, or glucocorticoids in any combination | Time to recurrence | Recurrence: 2/30 in rilonacept group and 23/31 in placebo group Time to recurrence: median time was 8.6 wks in placebo group and could not be calculated in rilonacept group (HR: 0.04 [95% CI: 0.01-0.18]; p<0.001) |
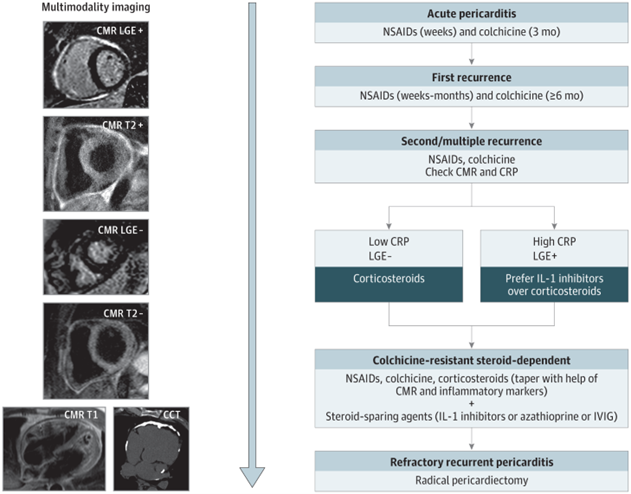
Non-steroidal anti-inflammatory drugs (NSAIDs) combined with colchicine is first-line treatment for patients with acute or recurrent pericarditis.4 Available NSAIDs include ibuprofen, indomethacin, and aspirin, which is preferred in patients with concomitant cardiovascular disease. Common NSAID side effects include gastroesophageal reflux disease (GERD) symptoms, peptic ulcer disease and nephrotoxicity.11 Multiple trials as shown in Figure 2 have proven the efficacy of colchicine in patients with acute as well as recurrent pericarditis. Adverse effects include gastrointestinal symptoms such as diarrhea and vomiting, weakness, alopecia, and polyneuropathy.11
Figure 2
Corticosteroids are typically reserved for patients with persistent symptoms despite the first-line therapies (NSAIDs and colchicine) or those with contraindications to NSAIDs/colchicine.4 Though they provide rapid relief of symptoms, there are three main concerns associated with corticosteroids; (1) increased risk of recurrence of pericarditis; (2) prolonging of disease course; (3) steroid-related side effects such as weight gain and muscle loss.3,12 When used, low to moderate dosages of 0.2-0.5 mg/kg/day are recommended to prevent recurrences and side effects.12,13 Immunosuppressive agents such as methotrexate, intravenous immunoglobulin (IVIG), and azathioprine are indicated as third-line agents for recurrent pericarditis.4 In light of limited evidence for their use, they are primarily used as steroid-sparing agents or to treat underlying auto-immune disease, if identified.11
The 2015 ESC guidelines gave a class IIb recommendation for the use of interleukin-1 (IL-1) inhibitor anakinra for the treatment of recurrent pericarditis.4 However, in the last 7 years, there has been tremendous growth in the data supporting the role of IL-1 inhibitors. Two recent randomized controlled trials, AIRTRIP and the RHAPSODY, strongly support the role of anakinra and rilonacept for the treatment of corticosteroid-dependent colchicine-resistant recurrent pericarditis.14,15 Rilonacept is the first Food and Drug Administration (FDA)-approved therapy for patients with recurrent pericarditis. By inhibiting IL-1 alpha and IL-1 beta, these biologics block the key component of the auto-inflammatory pathway responsible for the inflammatory phenotype of recurrent pericarditis.16 This not only leads to symptom relief by decreasing active inflammation but also decreases the likelihood of developing future recurrences.17,18 However, rilonacept is only studied in idiopathic and post pericardiotomy subgroups and the utility is not confirmed in other etiologies of pericarditis such as autoimmune or neoplastic. The major drawback of these agents is their prohibitive cost, which has inhibited their widespread use. Adverse effects include injection site reactions, upper respiratory and urinary tract infections, and hyperlipidemia.
Radical pericardiectomy is recommended for patients with refractory, treatment-resistant recurrent pericarditis.4 Though most patients report resolution of symptoms, some have persistent chest pain after surgery, presumably due to inflammation in residual pericardial tissue.
The Paradigm Shift
Traditionally, the decision of initiating or discontinuing medications for recurrent pericarditis has been made based on patient symptoms and serum inflammatory biomarkers. However, these inflammatory markers may be falsely negative in patients taking multiple anti-inflammatory therapies.19 Emerging data support modifying therapies in response to CMR findings and starting taper only once there is a significant improvement in the pericardial LGE and edema. A retrospective study of 507 recurrent pericarditis patients showed that the use of such a CMR-guided therapeutic approach decreased rates of recurrence, exposure to glucocorticoids, and pericardiocentesis.20 However, selection of patients for follow-up CMR is dependent on multiple factors such as response to therapy, clinical improvement, and inflammatory markers. A standardized approach is required, and evidence-based studies are warranted.
Given the proven efficacy of IL-1 inhibitors for patients with corticosteroid-dependent colchicine-resistant recurrent pericarditis with inflammatory phenotype and the clinical experience of high-volume centers, a new state-of-the-art treatment algorithm was proposed. This recommends earlier use of IL-1 inhibitors in patients with the inflammatory phenotype of pericarditis (Figure 2).11 Figure 2 integrates advanced imaging with emerging therapies for the management of patients with recurrent pericarditis. Figure 1 shows a patient with persistent pericardial inflammation who required the addition of rilonacept.
Conclusion
Recurrent pericarditis is a chronic debilitating condition complicating up to one-third of acute pericarditis cases. With CMR guidance, clinicians can better diagnose, prognosticate, and develop tailored treatment strategies for these patients. The remarkable efficacy of IL-1 inhibitors is a leap forward in the treatment of pericarditis. Such advances in imaging and targeted therapies have led to a paradigm shift in the evaluation and management of patients with recurrent pericarditis.
References
- Mody P, Bikdeli B, Wang Y, Imazio M, Krumholz HM. Trends in acute pericarditis hospitalizations and outcomes among the elderly in the USA, 1999-2012. Eur Heart J Qual Care Clin Outcomes 2018;4:98-105.
- Imazio M, Spodick DH, Brucato A, Trinchero R, Adler Y. Controversial issues in the management of pericardial diseases. Circulation 2010;121:916-28.
- Brucato A, Brambilla G, Moreo A, et al. Long-term outcomes in difficult-to-treat patients with recurrent pericarditis. Am J Cardiol 2006;98:267-71.
- Adler Y, Charron P, Imazio M, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the task force for the diagnosis and management of pericardial diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921-64.
- Kumar A, Sato K, Verma BR, et al. Quantitative assessment of pericardial delayed hyperenhancement helps identify patients with ongoing recurrences of pericarditis. Open Heart 2018;5:e000944.
- Klein AL, Abbara S, Agler DA, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2013;26:965-1012.e15.
- Imazio M, Pivetta E, Palacio Restrepo S, et al. Usefulness of cardiac magnetic resonance for recurrent pericarditis. Am J Cardiol 2020;125:146-51.
- Francone M, Carbone I, Agati L, et al. Utility of T2-weighted short-tau inversion recovery (STIR) sequences in cardiac MRI: an overview of clinical applications in ischaemic and non-ischaemic heart disease. Radiol Med 2011;116:32-46.
- Taylor AM, Dymarkowski S, Verbeken EK, Bogaert J. Detection of pericardial inflammation with late-enhancement cardiac magnetic resonance imaging: initial results. Eur Radiol 2006;16:569-74.
- Conte E, Agalbato C, Lauri G, et al. Cardiac MRI after first episode of acute pericarditis: a pilot study for better identification of high risk patients. Int J Cardiol 2022;354:63-67.
- Kumar S, Khubber S, Reyaldeen R, et al. Advances in imaging and targeted therapies for recurrent pericarditis: a review. JAMA Cardiol 2022;7:975-85.
- Lotrionte M, Biondi-Zoccai G, Imazio M, et al. International collaborative systematic review of controlled clinical trials on pharmacologic treatments for acute pericarditis and its recurrences. Am Heart J 2010;160:662-70.
- Imazio M, Brucato A, Cumetti D, et al. Corticosteroids for recurrent pericarditis: high versus low doses: a nonrandomized observation. Circulation 2008;118:667-71.
- Klein AL, Imazio M, Cremer P, et al. Phase 3 trial of interleukin-1 trap rilonacept in recurrent pericarditis. N Engl J Med 2021;384:31-41.
- Brucato A, Imazio M, Gattorno M, et al. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: the AIRTRIP randomized clinical trial. JAMA 2016;316:1906-12.
- Peet CJ, Rowczenio D, Omoyinmi E, et al. Pericarditis and autoinflammation: a clinical and genetic analysis of patients with idiopathic recurrent pericarditis and monogenic autoinflammatory diseases at a national referral center. J Am Heart Assoc 2022;11:e024931.
- Lopalco G, Rigante D, Cantarini L, et al. The autoinflammatory side of recurrent pericarditis: enlightening the pathogenesis for a more rational treatment. Trends Cardiovasc Med 2021;31:265-74.
- Imazio M, Andreis A, Piroli F, et al. Anti-interleukin 1 agents for the treatment of recurrent pericarditis: a systematic review and meta-analysis. Heart 2021;Mar 18[Epub ahead of print].
- Kumar A, Sato K, Yzeiraj E, et al. Quantitative pericardial delayed hyperenhancement informs clinical course in recurrent pericarditis. JACC Cardiovasc Imaging 2017;10:1337-46.
- Alraies MC, AlJaroudi W, Yarmohammadi H, et al. Usefulness of cardiac magnetic resonance-guided management in patients with recurrent pericarditis. Am J Cardiol 2015;115:542-47.
Clinical Topics: Cardiac Surgery, Cardiovascular Care Team, Dyslipidemia, Invasive Cardiovascular Angiography and Intervention, Pericardial Disease, Statins
Keywords: Pericarditis, Constrictive, Pericardial Effusion, C-Reactive Protein, Contrast Media, Constriction, Depression, Friction, Gadolinium, Pericarditis, Inflammation, Electrocardiography, Edema, Hospitalization, Azathioprine, Glucocorticoids, Immunoglobulins, Intravenous, Pericardiectomy, Interleukin 1 Receptor Antagonist Protein, Ibuprofen, Colchicine, Anti-Inflammatory Agents, Non-Steroidal, Aspirin, Methotrexate, Indomethacin, Interleukin-1alpha, Immunosuppressive Agents, Interleukin-1beta, Pericardiocentesis, Biological Products, Cardiovascular Diseases, United States Food and Drug Administration, Retrospective Studies, Hyperlipidemias, Follow-Up Studies, Patient Selection, Peptic Ulcer, Phenotype, Biomarkers, Gastroesophageal Reflux, Contraindications, Algorithms, Immune System Diseases
< Back to Listings