Anticoagulation Algorithm For Fontan Patients
Quick Takes
- Antithrombotic prophylaxis is recommended in all Fontan patients due to the high incidence of thromboembolic events.
- Aspirin, warfarin, and non-vitamin K antagonist oral anticoagulants (NOACs) are all effective for thromboprophylaxis in Fontan patients, with recent studies indicating the safety and efficacy of NOACs.
- Cardiologists should routinely assess patient's individual thromboembolic risk profile and adjust anticoagulation strategies accordingly, with options ranging from antiplatelet therapy to oral vitamin K antagonist or NOAC therapy based on the risk level.
Patients with functionally univentricular hearts have greatly improved survival prospects thanks to the Fontan palliation, which routes systemic venous blood directly to the pulmonary vascular bed with the lack of a subpulmonic pump.1 This approach leads to significant circulatory challenges resulting in short- and long-term complications due to chronic low cardiac output, high central venous pressure, and venous stasis. Thrombus formation and thromboembolic events are significant adverse outcomes associated with the Fontan circulation, which can lead to complications such as Fontan circuit obstruction and pulmonic or paradoxical emboli.2 The Fontan technique increases the risk of thrombosis due to various factors, such as low-velocity nonlaminar flow, prosthetic material, endothelial dysfunction, blind-ending pouches, atrial arrhythmias, and altered procoagulant and anticoagulant factors. Consequently, there is a consensus on the need for antithrombotic prophylaxis in all Fontan patients to reduce the high incidence of thromboembolic events.
Previous studies suggest that aspirin, warfarin, and non-vitamin K antagonist oral anticoagulants (NOACs) all provide benefits for thromboprophylaxis in Fontan patients.3-7 A recent network meta-analysis has indicated that the risk of thromboembolic events can indeed be reduced with the use of aspirin, warfarin, and NOACs.8 Despite the limited number of patients and variations in studies using NOACs, the findings indicate a favorable safety and efficacy profile for the use of NOACs in individuals with a Fontan circulation. Based on the emerging data supporting the use of NOACs in Fontan patients, many Fontan management programs – including our program – have begun to adjust their thromboprophylaxis strategies to include NOACs as an option. Below is an example of a recent protocol developed by our team.
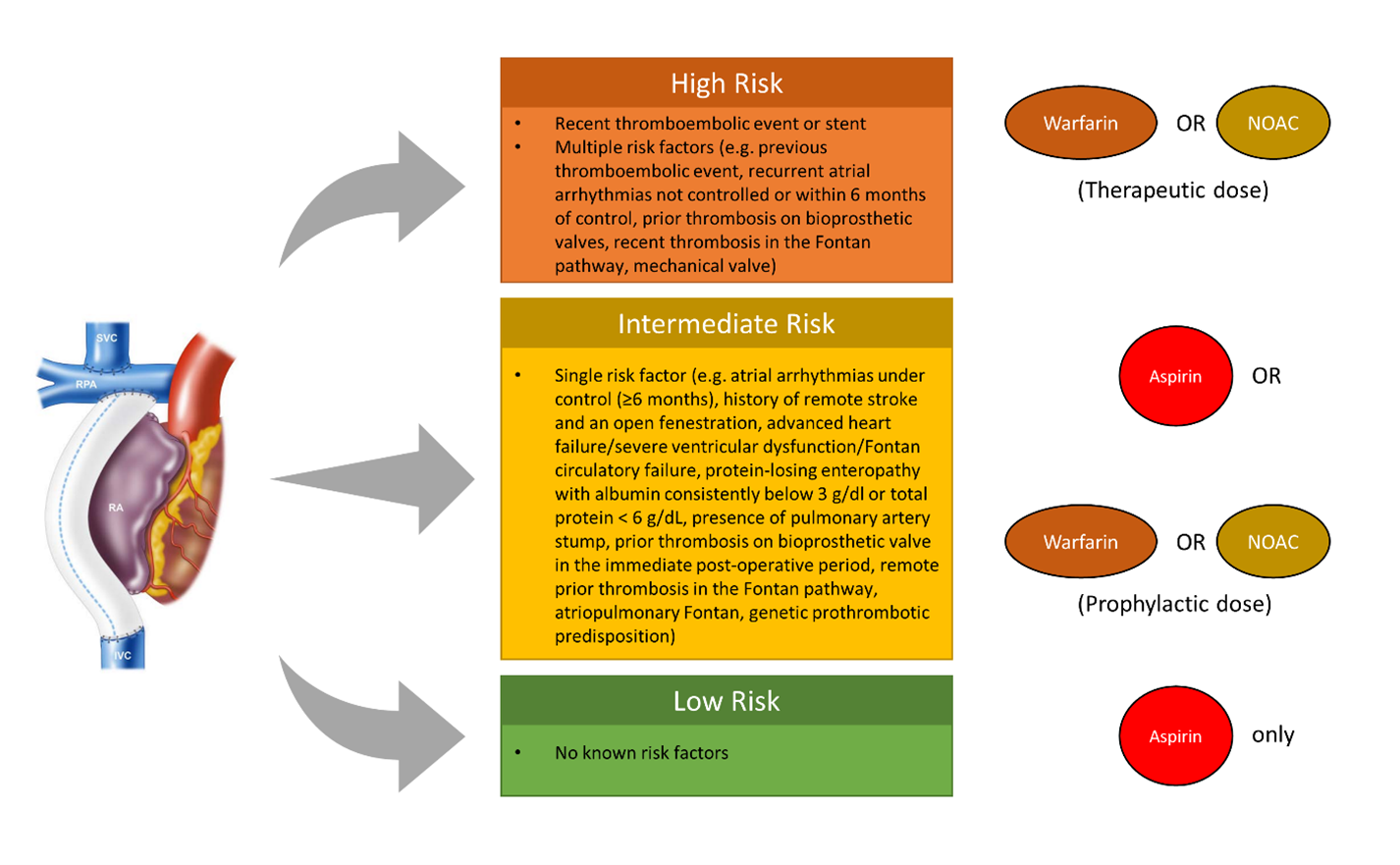
Step 1: Assess the Individual Thromboembolic Risk Profile in Fontan Patients
- High risk (anticoagulation necessary): Recent thromboembolic event, mechanical valve or recent stent placement or other risk factors (e.g., previous thromboembolic event, recurrent atrial arrhythmias not controlled or within 6 months of control, prior thrombosis on bioprosthetic valves, recent thrombosis in the Fontan pathway within 6 months).
- Intermediate risk (reasonable to use anticoagulation): Atrial arrhythmias under control (≥6 months), history of remote stroke and an open fenestration, advanced heart failure/severe ventricular dysfunction/Fontan circulatory failure, protein-losing enteropathy with albumin consistently below 3 g/dl or total protein < 6 g/dL, presence of pulmonary artery stump, prior thrombosis on bioprosthetic valve in the immediate post-operative period, remote prior thrombosis in the Fontan pathway, atriopulmonary Fontan, genetic prothrombotic predisposition.
- Low risk (antiplatelet only): None of the risk factors described above.
Step 2: Select Anticoagulation Strategy
- High risk (anticoagulation necessary): Start anticoagulation with oral vitamin K antagonist (e.g., warfarin) or therapeutic dose of NOAC.
- Intermediate risk (reasonable to use anticoagulation): Antiplatelet therapy with aspirin or consider anticoagulation with oral vitamin K antagonist or prophylactic dose of NOAC.
- Low risk (antiplatelet only): No anticoagulation needed. Antiplatelet therapy with aspirin only.
Step 3: Monitor and Adjust Therapy
- For patients on oral vitamin K antagonist therapy: Monitor international normalized ratio (INR) and adjust the dose as needed to maintain INR in the therapeutic range (typically 2-3).
- For patients on NOAC therapy: No routine monitoring of the anticoagulant effect is needed but consider periodic monitoring of renal function and drug levels in certain situations (e.g., chronic kidney disease).
- For all patients: Reassess thromboembolic and bleeding risk periodically and adjust anticoagulation strategy as needed.
A few additional notes to consider:
- Avoidance of modifiable risk factors should be considered the first and foremost intervention. Patients should refrain from smoking and avoid use of estrogen containing oral contraceptives. Additionally, physical inactivity should be avoided.
- Low molecular weight heparin can be used in a few scenarios when patients cannot achieve therapeutic INR and NOACs are not available.
- The aspirin antiplatelet dose is 1-5 mg/kg to a maximum of 325 mg per day.6
- In exceptional scenarios a combination of anticoagulation and antiplatelet has anecdotally been used (e.g., adding aspirin to a high risk patient with Fontan who is on anticoagulation at baseline after stent placement).9
- There is a lower threshold to use anticoagulation in older adults as they traditionally have a higher risk profile for thrombosis.3
- The recommended doses of one of the NOACs (rivaroxaban) have been published as part of the UNIVERSE trial in a pediatric population.6
- Note that NOACs are contraindicated in pregnancy and in patients with mechanical valves. The embryo- and fetotoxic effect of warfarin during pregnancy is dose-dependent. The decision for the use of warfarin or conversion to low-molecular-weight heparin or aspirin during pregnancy must be based on the thromboembolic risk, bleeding risk and the risk of adverse fetal events.
- While some preprocedural antidotes for NOACs have become available (i.e., idarucizumab for dabigatran, andexanet alfa for rivaroxaban and apixaban), these are not universally available and/or limited by cost/safety concerns. This should be taken into consideration in patients who may require urgent major surgery (e.g., listed for transplantation) where warfarin is typically used. For elective major surgery, NOACs can be stopped 24-48 hours before (based on the type of NOAC and kidney function) without the need for bridging with low molecular weight heparin.
- It is important to note the relatively small number of patients treated with NOACs in the medical literature, and the relatively short follow up time. Also the cost of NOAC should be taken into consideration.10
- It is vital to consider the benefits of anticoagulation with warfarin against the challenges of frequent INR testing, dietary and medication interactions, potential issues with compliance, and bleeding risks in patients participating in high-risk activities. Although NOACs do not eliminate all of these challenges, they do remove the burden of frequent INR testing and dose adjustments. In young children achieving a therapeutic INR can be very difficult and NOACs may be an attractive alternative in this scenario. We have observed increased use of NOACs in this patient population, and future studies will provide further insight into the optimal strategy for selecting appropriate thromboprophylaxis.10
Figure 1
References
- Rychik J, Atz AM, Celermajer DS, et al; American Heart Association Council on Cardiovascular Disease in the Young and Council on Cardiovascular and Stroke Nursing. Evaluation and management of the child and adult with Fontan circulation: a scientific statement from the American Heart Association. Circulation 2019;Jul 1:[ePub ahead of print].
- Attard C, Huang J, Monagle P, Ignjatovic V. Pathophysiology of thrombosis and anticoagulation post Fontan surgery. Thromb Res 2018;172:204-13.
- Small AJ, Aboulhosn JA, Lluri G. Thromboprophylaxis in adults with atrio-pulmonary Fontan. World J Pediatr Congenit Heart Surg 2018;9:504-08.
- Yang H, Veldtman GR, Bouma BJ, et al. Non-vitamin K antagonist oral anticoagulants in adults with a Fontan circulation: are they safe. Open Heart 2019;Jun 3:[ePub ahead of print].
- Al-Jazairi AS, Al Alshaykh HA, Di Salvo G, De Vol EB, Alhalees ZY. Assessment of late thromboembolic complications post-Fontan procedure in relation to different antithrombotic regimens: 30-years' follow-up experience. Ann Pharmacother 2019;53:786-93.
- McCrindle BW, Michelson AD, Van Bergen AH, et al; UNIVERSE Study Investigators. Thromboprophylaxis for children post-Fontan procedure: insights from the UNIVERSE study. J Am Heart Assoc 2021;Sep 24:[ePub ahead of print].
- Kawamatsu N, Ishizu T, Machino-Ohtsuka T. Direct oral anticoagulant use and outcomes in adult patients with Fontan circulation: a multicenter retrospective cohort study. Int J Cardiol 2021;327:74-79.
- Van den Eynde J, Possner M, Alahdab F, et al. Thromboprophylaxis in patients with Fontan circulation. J Am Coll Cardiol 2023;81:374-89.
- Miwa K, Iwai S, Nagashima T. Anticoagulation therapy after the Fontan procedure. Pediatr Cardiol 2022;43:1271-76.
- Lin J, Lluri G. Thromboprophylaxis in the Fontan circulation. J Am Coll Cardiol 2023;81:390–93.
Clinical Topics: Anticoagulation Management, Arrhythmias and Clinical EP, Cardiac Surgery, Congenital Heart Disease and Pediatric Cardiology, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Pulmonary Hypertension and Venous Thromboembolism, Vascular Medicine, Anticoagulation Management and Venothromboembolism, Implantable Devices, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Cardiac Surgery and Arrhythmias, Cardiac Surgery and CHD and Pediatrics, Cardiac Surgery and Heart Failure, Congenital Heart Disease, CHD and Pediatrics and Arrhythmias, CHD and Pediatrics and Interventions, CHD and Pediatrics and Prevention, CHD and Pediatrics and Quality Improvement, Novel Agents, Interventions and Structural Heart Disease, Interventions and Vascular Medicine
Keywords: Anticoagulants, Warfarin, Fontan Procedure, International Normalized Ratio, Rivaroxaban, Dabigatran, Antidotes, Fibrinolytic Agents, Heparin, Low-Molecular-Weight, Aspirin, Protein-Losing Enteropathies, Central Venous Pressure, Contraceptives, Oral, Administration, Oral, Univentricular Heart, Venous Thromboembolism, Thrombosis, Arrhythmias, Cardiac, Renal Insufficiency, Chronic, Estrogens, Albumins, Stents, Meta-Analysis as Topic
< Back to Listings