Quantitative Coronary Plaque Analysis by Coronary Computed Tomography Angiography: Is it Ready For Primetime?
Quick Takes
- Quantitative coronary plaque analysis (QCPA) is an emerging tool that allows noninvasive quantification and characterization of coronary atherosclerosis on the basis of coronary computed tomography angiography (CCTA) findings.
- QCPA can enhance risk stratification and may ultimately provide a method to assess response to various medical therapies in clinical research or in practice.
- A recent expert consensus document from the Society of Cardiovascular Computed Tomography (SCCT) establishes new standards for quantitative assessments using CCTA, offering detailed expert guidance on acquisition, analysis, reporting, and interpretation.
A unique strength of coronary computed tomography angiography (CCTA) is the ability to noninvasively detect nonobstructive and obstructive coronary plaque.1 Qualitative coronary plaque analysis and quantitative coronary plaque analysis (QCPA) via CCTA has been shown to enhance patient risk stratification, intensify preventive therapies, and improve patient outcomes.2,3 Accordingly, the updated Coronary Artery Disease Reporting–Data System (CAD-RADS™ 2.0 [SCCT, Arlington, Virginia]) emphasizes that every CCTA report should provide an estimate of overall plaque burden, and that this information should be used in providing clinical recommendations.1 Although these recommendations suggest various semiquantitative methods that can be used to estimate plaque burden, quantitative plaque analysis is likely to become more widely available.
Advances in computational power and artificial intelligence have reduced the time for QCPA and have improved its reproducibility. However, challenges remain, including lack of standardization in acquisition, differing methods for plaque subtype detection, and the absence of consensus on reporting and interpretation. The Society of Cardiovascular Computed Tomography (SCCT) recently published an expert consensus document on standards for quantitative assessments by CCTA.4 This expert analysis highlights the key points addressed by this consensus document and identifies remaining knowledge gaps.
Image Acquisition, Quality, and Analysis
The consensus document emphasizes the importance of high-quality image acquisition by optimizing spatial and temporal resolution and minimizing noise and artifacts to ensure accurate and reproducible QCPA. Table 1 presents the primary recommendations for achieving these goals. Modifying computed tomography (CT) parameters has the potential to significantly affect image quality and plaque quantification. For instance, using a higher tube current reduces image noise and improves reproducibility. In contrast, using a lower tube voltage or a higher iodine concentration increases lumen attenuation and can raise the attenuation values of all plaque components. This can lead to higher calcified plaque (CP) and lower low-attenuation plaque (LAP) volumes. Sharper reconstruction kernels improve contour clarity but increase noise, whereas using iterative reconstruction reduces noise.4
Table 1: Select Recommendations for Image Acquisition, Quality, and Plaque Analysis*
| Optimizing spatial resolution |
|
| Optimizing temporal resolution |
|
| Reducing image noise |
|
| Minimizing artifacts |
|
| Plaque analysis |
|
CT = computed tomography; ECG = electrocardiogram; HR = heart rate; IV = intravenous; IVUS = intravascular ultrasound; NTG = nitroglycerin.
When assessing plaque burden, one important concept is that the identification and quantification of different types of plaque morphology have variable diagnostic accuracy and reproducibility. For instance, quantifying CP is more reproducible but may offer less clinical value, especially for assessing response to various therapies. In contrast, the detection of LAP has been shown to be prognostically important,5 but may be less reproducible.6 Currently, overall plaque volume is the most commonly assessed plaque measure to assess prognosis, whereas the quantification of noncalcified plaque (NCP) volume may be the most meaningful parameter to follow for assessing response to various therapies.
The use of semiautomated or automated lumen and vessel segmentation is preferred over manual analysis for its greater reproducibility and practicality (Figure 1). However, different quantitative techniques may perform differently and vary in terms of validation. Despite advancements, having a human-in-the-loop to provide oversight and correction frequently remains necessary.7
Figure 1: Main Steps in Quantitative Plaque Analysis by Computed Angiography Tools
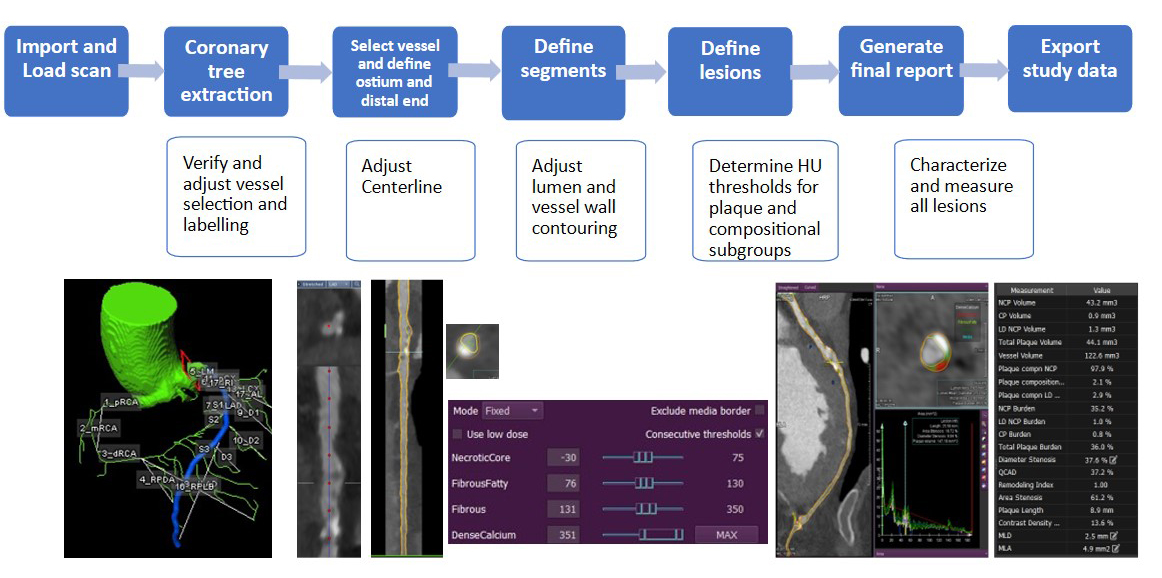
HU = Hounsfield unit.
Serial Scanning and CCTA-Derived Quantitative Plaque Endpoints
Temporal changes in plaque quantity and morphology, assessed by serial CCTA, can serve as surrogate imaging endpoints to evaluate the efficacy of various therapies. Key considerations for selecting a CCTA-derived plaque outcome include ensuring that the CT variable is likely to be affected by the intervention under study and that any changes in the imaging endpoint are clinically significant. The endpoint should ideally be specific, demonstrate consistent changes, and exhibit low temporal variability within individuals.4
To enhance reproducibility, it is important to maintain uniformity in CT platforms and scanning parameters, as well as to maintain consistency in the method of analysis used for a given patient.
Nomenclature
The consensus document standardizes the nomenclature, definitions, and reporting units for key CCTA-derived parameters used to measure lumen, vessel, and plaque dimensions.
For instance, the traditional definition of plaque on CCTA is the presence of tissue structures ≥1 mm2 within or adjacent to the coronary artery lumen, identified in at least two independent planes, that can be distinguished from the surrounding tissues (epicardial fat) and the lumen. Furthermore, plaque burden (%) is defined as the proportion of plaque relative to the overall vessel size, and total plaque volume (mm3) is defined as the difference between the vessel and lumen volumes in the studied region. Importantly, these CCTA-based definitions may differ from those used in invasive coronary angiography and plaque imaging.
Assessment and Classifications of Plaque
The consensus document advises against using plaque classifications that imply mechanical or histological characteristics not reliably assessed by CCTA, such as soft, hard, mixed, vulnerable, lipid-rich, and fibrous plaque. The CCTA-derived plaque classification is based on attenuation. Two primary threshold methods are employed: the fixed method and the adaptive method. For the fixed method, although universally accepted thresholds have not been established, the suggested categories include2,8:
- LAP: -30 to 30 Hounsfield units (HU; or -30 to 75 HU)
- Fibrofatty plaque: 31-130 HU
- Fibrous plaque: 131-350 HU
- CP: >350 HU
The adaptive method adjusts plaque thresholds on the basis of specific image characteristics.8 Both methods are feasible and are offered by various vendors. However, differences in plaque volumes are expected depending on the method chosen (Figure 2).
Figure 2: The Effect of HU Thresholds on Quantitative Plaque Measurements

CP = calcified plaque; HU = Hounsfield units; LAP = low-attenuation plaque; NCP = noncalcified plaque; TPV = total plaque volume.
Pragmatic age-stratified and sex-stratified percentile nomograms for atherosclerotic plaque measurements from QCPA have been reported and can be used to contextualize findings for individual patients.9
QCPA in Clinical Practice
Although clinical indications are not yet fully defined, QCPA can help refine prognosis and personalize preventive therapies, especially among individuals with nonobstructive coronary artery disease (CAD) (Figure 3; Video 1). Patients with extensive nonobstructive CAD may be at a substantially higher risk than those who have no or minimal CAD, yet their risk is often overlooked and thus they may be undertreated.10 Other potential subgroups of patients for whom QCPA may be helpful include those with a large burden of NCP (e.g., patients with inflammatory diseases, HIV, or diabetes mellitus).
Figure 3: Example of Quantitative Coronary Plaque Analysis
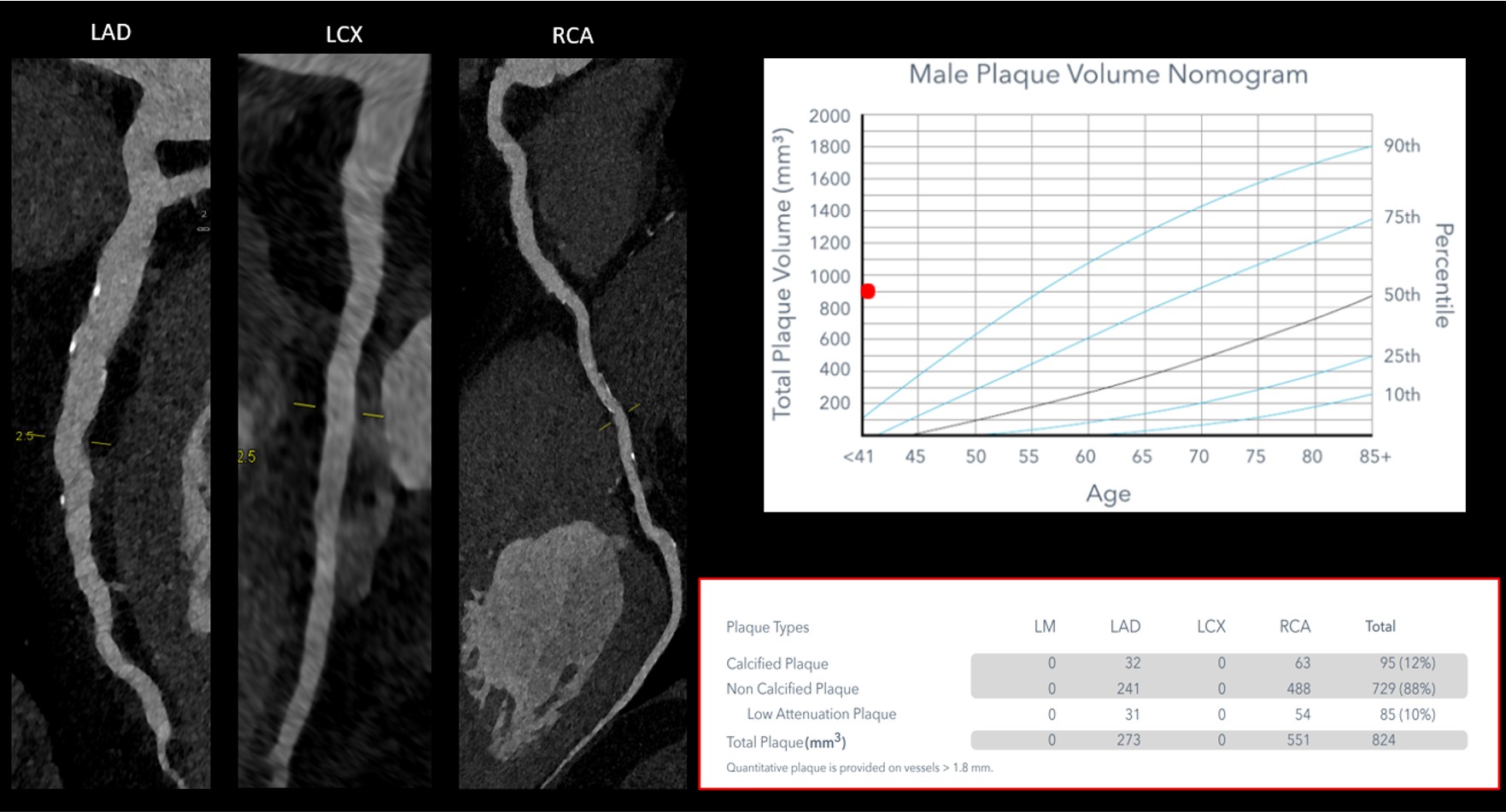
CCTA and QCPA were performed on a 32-year-old man with elevated Lp(a) levels (230 nmol/L) to evaluate nonspecific chest discomfort. Although no obstructive CAD was identified, a significant burden of both CP and NCP was observed, as shown in the 3D color-coded model (Video 1), along with the quantitative table and nomogram (this figure). This finding prompted administration of aggressive preventive therapies.
3D = three-dimensional; CAD = coronary artery disease; CCTA = coronary computed tomography angiography; CP = calcified plaque; Lp(a) = lipoprotein(a); NCP = noncalcified plaque; QCPA = quantitative coronary plaque analysis.
Video 1: 3D Color-Coded Model Showing Significant Burden of Both Calcified and Noncalcified Plaque
3D = three-dimensional; CAD = coronary artery disease; CCTA = coronary computed tomography angiography; CP = calcified plaque; Lp(a) = lipoprotein(a); NCP = noncalcified plaque; QCPA = quantitative coronary plaque analysis.
Quantitative Plaque Analysis in Clinical Research
Quantitative plaque CCTA can play an important role in research, serving either as a tool for risk enrichment or as a surrogate endpoint to assess the efficacy of various interventions. Examples of trials that illustrate this approach include the GOLDILOX (Efficacy and Safety of MEDI6570 in Patients With a History of Myocardial Infarction) and REMBRANDT (A CCTA Imaging Trial to Evaluate the Effect of Obicetrapib/Ezetimibe on Coronary Plaque) trials, both of which evaluate medical interventions and use quantitative plaque as a surrogate outcome. This approach could allow for the conduction of smaller feasibility studies before initiating a large-scale cardiovascular outcomes trial, or for gaining mechanistic insights into various treatments.
Such trials require careful planning, expert imaging sites, and a skilled core laboratory.
Future Directions and Gaps in Knowledge
Data linking HU thresholds with plaque morphology are limited. More research is needed to refine plaque classification thresholds, calibrate measurements across scanners, and define what constitutes a significant plaque change. Fully automated tools need further validation to improve accessibility, and more data are required to guide patient management and identify those who would benefit most from QCPA. Despite the aforementioned limitations, there is great potential for QCPA to improve how clinicians interpret and report CCTA findings, and ultimately to enhance how CCTA can guide the use of future therapies and improve patient outcomes.
Summary
The authors commend the SCCT and the contributors to this consensus document, which is an important step toward standardizing quantitative plaque analysis by CCTA and its application in clinical practice and research. Further evidence and research are needed to address the existing gaps and refine these recommendations.
References
- Cury RC, Leipsic J, Abbara S, et al. CAD-RADS™ 2.0 - 2022 Coronary Artery Disease-Reporting and Data System: an expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR), and the North America Society of Cardiovascular Imaging (NASCI). JACC Cardiovasc Imaging 2022;15:1974-2001.
- Shaw LJ, Blankstein R, Bax JJ, et al. Society of Cardiovascular Computed Tomography / North American Society of Cardiovascular Imaging - expert consensus document on coronary CT imaging of atherosclerotic plaque. J Cardiovasc Comput Tomogr 2021;15:93-109.
- Newby DE, Adamson PD, Berry C, et al.; SCOT-HEART Investigators. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med 2018;379:924-33.
- Nieman K, García-García HM, Hideo-Kajita A, et al. Standards for quantitative assessments by coronary computed tomography angiography (CCTA): an expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT). J Cardiovasc Comput Tomogr 2024;18:429-43.
- Wang XP, Zhang W, Liu XQ, et al. Arginase I enhances atherosclerotic plaque stabilization by inhibiting inflammation and promoting smooth muscle cell proliferation. Eur Heart J 2014;35:911-9.
- Narula J, Stuckey TD, Nakazawa G, et al. Prospective deep learning-based quantitative assessment of coronary plaque by computed tomography angiography compared with intravascular ultrasound: the REVEALPLAQUE study. Eur Heart J Cardiovasc Imaging 2024;25:1287-95.
- Koo BK, Yang S, Jung JW, et al. Artificial intelligence-enabled quantitative coronary plaque and hemodynamic analysis for predicting acute coronary syndrome. JACC Cardiovasc Imaging 2024;17:1062-76.
- de Graaf MA, Broersen A, Kitslaar PH, et al. Automatic quantification and characterization of coronary atherosclerosis with computed tomography coronary angiography: cross-correlation with intravascular ultrasound virtual histology. Int J Cardiovasc Imaging 2013;29:1177-90.
- Tzimas G, Gulsin GS, Everett RJ, et al. Age- and sex-specific nomographic CT quantitative plaque data from a large international cohort. JACC Cardiovasc Imaging 2024;17:165-75.
- Bienstock S, Lin F, Blankstein R, et al. Advances in coronary computed tomographic angiographic imaging of atherosclerosis for risk stratification and preventive care. JACC Cardiovasc Imaging 2023;16:1099-115.
Clinical Topics: Cardiovascular Care Team, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Atherosclerotic Disease (CAD/PAD), Interventions and Coronary Artery Disease, Interventions and Imaging, Angiography, Computed Tomography, Nuclear Imaging, Prevention
Keywords: Coronary Angiography, Computed Tomography Angiography, Coronary Artery Disease, Atherosclerosis
