Antimalarial-Mediated Cardiotoxicity: Evaluation and Preliminary Screening Recommendations For Patients on Chronic Therapy for Autoimmune Rheumatic Diseases
Quick Takes
- With the growing number of patients exposed to long-term antimalarial (AM) therapy for chronic autoimmune/inflammatory diseases, appropriate screening for cardiotoxicity associated with AM therapy is increasingly necessary to prevent the development of this potentially fatal complication.
- Clinicians should have a high index of suspicion for AM cardiotoxicity in patients at moderate or higher risk who present with new cardiac symptoms or abnormalities on regular cardiac screening/evaluations.
- In the absence of data from large randomized studies and strong clinical evidence, these preliminary screening recommendations and workup for AM cardiotoxicity are intended to serve as a potential guide to clinicians until formal screening recommendations can be developed and validated.
Case Presentation
A 65-year-old woman with a history of systemic lupus erythematosus (SLE) treated with prednisone and hydroxychloroquine 300 mg/day for 40 years, stage 3 chronic kidney disease, and hypertension presented to the cardiology clinic with progressive dyspnea on exertion.
Initial workup with transthoracic echocardiogram (TTE) revealed biventricular (BiV) systolic dysfunction with left ventricular ejection fraction (LVEF) 35%, basal septal hypertrophy, biatrial enlargement, and moderate mitral regurgitation. An electrocardiogram (ECG) had findings of first-degree atrioventricular block and nonspecific interventricular conduction delay. Left heart catheterization revealed no obstructive coronary artery disease. Cardiac magnetic resonance imaging (cMRI) had findings of notable progressive BiV dysfunction, with late gadolinium enhancement (LGE) in the midmyocardial wall consistent with a nonischemic process and a chronic myocardial infarction in the mid to distal left anterior descending artery territories (Figure 1), and cardiac positron emitted tomography had findings of infarction in the corresponding territory without notable cardiac inflammation. Follow-up TTE had findings of concentric left ventricular (LV) hypertrophy, LVEF 20%, and right ventricular (RV) enlargement with severely impaired RV function. She underwent endomyocardial biopsy (EMB), which had findings of myocyte hypertrophy, extensive myocyte vacuolization, and interstitial fibrosis consistent with antimalarial (AM) cardiotoxicity (Figure 2).
Figure 1: Cardiac MRI-LGE PSIR
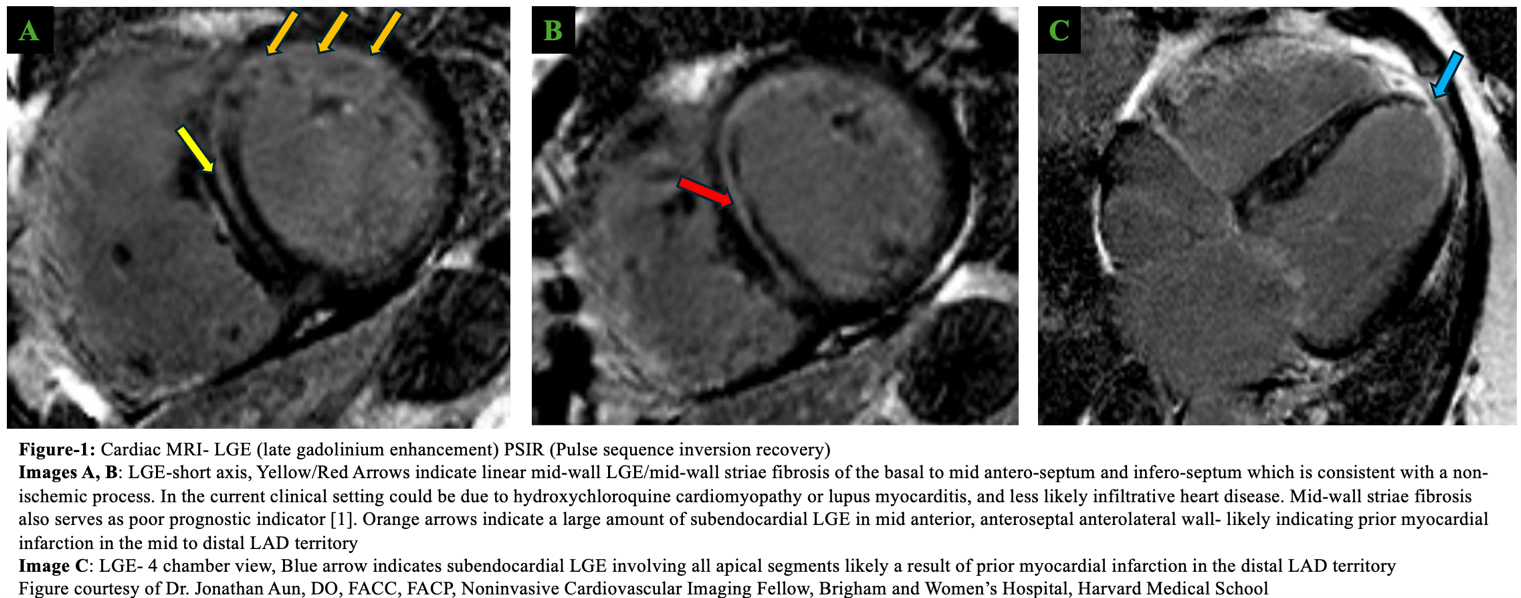
[1] Purmah et al. Sci Rep 2022;12:1739.1
LAD = left anterior descending coronary artery; LGE = late gadolinium enhancement; MRI = magnetic resonance imaging; PSIR = pulse sequence inversion recovery.
Figure 2: Pathology Endomyocardial Biopsy

Despite AM discontinuation and guideline-directed medical therapy (GDMT), she continues to decline over the course of several months and ultimately dies from complications of congestive heart failure (CHF).
Discussion
Hydroxychloroquine and chloroquine derivatives are AM drugs that continue to be cornerstone treatments for chronic systemic autoimmune diseases such as SLE and rheumatoid arthritis (RA). AM agents have multiple beneficial effects in these diseases, including reduced disease flares, thrombotic events, tissue damage accrual, and mortality. The cardiovascular (CV) benefits of AM agents in patients with SLE and RA include lower prevalence of atherosclerotic CV disease and risk factors.2,3 AM drugs are thought to exert their effects via interference with lysosomal activity, autophagy, and inhibition of cytokine production through distortion of membrane-signaling pathways, resulting in blockade of proinflammatory transcriptional activity. AM agents have large volumes of distribution and extended half-lives, preferentially accumulating in acidic compartments such as lysosomes and inflamed acidic tissues. This phenomenon contributes to the known ophthalmologic and neuromuscular toxicities, as well as to the risk of cardiotoxicity, which typically presents in advanced stages as a hypertrophic restrictive cardiomyopathy (similar to the cardiomyopathy of Anderson-Fabry disease) and is a consequence of drug accumulation within lysosomes of cardiomyocytes. Table 1 outlines potential presentations of AM-mediated cardiotoxicity.4-6
Table 1: Clinical Manifestations of Antimalarial-Mediated Cardiotoxicity
|
Cardiac Biomarker Elevation
|
Conduction System Disease
|
Structural Heart Disease
|
Diastolic Dysfunction
|
Systolic Dysfunction
|
|
| Presenting symptoms | Often asymptomatic | Varies from asymptomatic to palpitations, syncope, and sudden death | Varies from asymptomatic to ≥1 symptoms of L-sided or R-sided CHF | ||
| Clinical significance | Early sign of myocardial cell injury and cellular dysfunction | May increase risk of SCD, presyncope/syncope, and exacerbations of CHF | May precede symptoms and systolic and diastolic dysfunction May accompany new valvular disease |
May precede systolic dysfunction May initially be asymptomatic |
Later stages of disease May be reversible or stabilized in some cases with optimal GDMT and drug discontinuation but may also be progressive |
| Testing modalities | hs-Trop NT-BNP |
ECG Ambulatory cardiac monitor |
TTE cMRI PYP scan |
TTE cMRI RHC |
TTE cMRI |
| Characteristic findings on diagnostic evaluationa | Persistent elevation in cardiac biomarker level(s) May accompany elevations in CPK levels (representing parallel neuromyotoxicity) |
Varying degrees of AV block (1st-3rd) (4-6%) LAFB, complete/ incomplete LBBB or RBBB, widened QRS (13-15%) AF/AFL (2-3%) QTc prolongation (8-10%) VAs including TdP (2%) Conduction system disease cumulatively (85%) |
Typically HCM ± RCM and may include: Ventricular hypertrophy LV ± RV, concentric (22%) IVS thickening >11 mm Biatrial enlargement New valvular disease: SAM, MR 2/2 papillary muscle disruption (2-7%) Nonischemic patchy areas of LGE on cMRI (fibrosis), T1 mapping abnormalities indicate fibrosis typically in nonvascular territories (Figure 2, panels F-H) Can present with false-positive cardiac-PYP scan findings Absence of characteristic findings of myocarditis, sarcoidosis, amyloidosis, hypertensive CM, and HCM on cMRI, although multiple processes may be present |
Impaired LV relaxation (16-20%) Restrictive filling pattern Increased E/A ratio Shortened DT Increased E/e′ ratio Increased LV filling pressures, may be confirmed with invasive hemodynamics |
Nonischemic territory hypokinesis (9-10%) Reduced GLS patterns, especially in the IVS and mid lateral wall LV systolic dysfunction/ reduced EF (35%) RV systolic dysfunction (4-5%) |
| Confirmatory diagnostic/reference standard | EMB with histologic examination demonstrates interstitial fibrosis, enlarged vacuolated cells with central nuclei. Electron microscopy demonstrates characteristic cytoplasmic inclusion bodies with pathognomonic curvilinear and lamellar myelin bodies. (Figure 1, panels A-C; Figure 2, panels D and E) | ||||
AF = atrial fibrillation; AFL = atrial flutter; AV = atrioventricular; CHF = congestive heart failure; CM = cardiomyopathy; cMRI = cardiac magnetic resonance imaging; CPK = creatine phosphokinase; DT = deceleration time; ECG = electrocardiogram; EF = ejection fraction; EMB = endomyocardial biopsy; GDMT = guideline-directed medical therapy; GLS = global longitudinal strain; HCM = hypertrophic cardiomyopathy; hs-Trop = high-sensitivity troponin; IVS = interventricular septum; L = left; LAFB = left anterior fascicular block; LBBB = left bundle branch block; LGE = late gadolinium enhancement; LV = left ventricular; MR = mitral regurgitation; NT-BNP = N-terminal B-type natriuretic peptide; PYP = pyrophosphate; R = right; RBBB = right bundle branch block; RCM = restrictive cardiomyopathy; RHC = right heart catheterization; RV = right ventricular; SAM = systolic anterior motion; SCD = sudden cardiac death; TdP = torsade de pointes; TTE = transthoracic echocardiogram; VA = ventricular arrhythmia.
Preliminary Expert Opinion–Based Screening Practices
As cumulative lifetime AM dose increases, so does the risk of cardiotoxicity, although the exact threshold of toxic exposure is unknown. Although cardiotoxicity among patients taking chronic AM therapy was historically considered rare, more recent reports indicate that this cardiotoxicity may be underestimated, although the exact prevalence remains unknown because of a paucity of data. Biomarker screening in patients with SLE without pulmonary hypertension or previous cardiac disease has indicated that 10.6% of patients had incidental elevation in cardiac biomarker levels, and a third of these patients were confirmed to have AM cardiotoxicity.7 Prognosis after AM discontinuation varies widely. Although some patients clinically improve (60%), many continue to decline despite drug discontinuation and require cardiac pacemaker placement (7%), require transplant (1%), or die (27%).4 Case series data have shown that LGE and LV dysfunction may continue to progress long after drug discontinuation.8
Without established screening practices for patients taking chronic AM therapy, most reports of AM cardiotoxicity are identified after symptoms have developed and structural damage is evident on CV evaluation. The use of AM as a first-line disease-modifying drug in chronic autoimmune disorders, as well as the potentially irreversible and devastating consequences of cardiotoxicity with long-term use, necessitate the creation of CV screening protocols to allow early detection of cardiotoxicity.
Recommended annual retinal screenings are a currently well-established practice for patients taking chronic AM therapy due to the association with retinopathy in 5-20% of patients depending on the therapy duration.9 A similar strategy is needed to enhance early detection of AM cardiotoxicity, allowing appropriate and timely drug discontinuation to reduce AM-induced cardiac disease. Unfortunately, no large studies have been undertaken to provide clear guidance on optimal cardiac screening methodology for patients taking chronic AM therapy. Limited study data have demonstrated evidence that subclinical abnormalities including cardiac biomarker elevation, ECG conduction system disease, and echocardiographic abnormalities develop before symptomatic cardiotoxicity, providing a window of opportunity to prevent progression to potentially irreversible, symptomatic disease.4,7,8,10
Risk-based, preliminary, screening protocols that are recommended for patients taking chronic AM therapy are outlined in Table 2. In the absence of strong prospective data, these protocols are suggestions to increase awareness and guide clinicians until formal screening recommendations can be developed and validated with prospective studies.
Table 2: Risk Factors for Antimalarial-Mediated Cardiotoxicity
1. Patients receiving higher doses and/or longer durations
|
| 2. Patients with pre-existing cardiac conditions (SHD, IHD, myocarditis) |
| 3. Patients with kidney insufficiency/CKD (due to reduced drug clearance) |
| 4. Patients taking concomitant NSAIDs |
| 5. Patients with ocular, dermatologic, or skeletal muscle toxicity are at automatic high risk of concomitant CV toxicity |
| Preliminary recommended screening practices for patients taking chronic AM therapy |
Prior to AM drug initiation:
|
1. Lower Risk (1 Risk Factor)a:
|
2. Moderate Risk (2-3 Risk Factors)b:
|
3. Higher Risk (4+ Risk Factors)c:
|
bFor example, cumulative dose ≥500,000 mg, duration ≥5 years, or kidney insufficiency
cFor example, cumulative dose ≥500,000 mg, duration ≥5 years, history of MI, and kidney insufficiency
AF = atrial fibrillation; AFL = atrial flutter; AM = antimalarial; AV = atrioventricular; CKD = chronic kidney disease; CV = cardiovascular; ECG = electrocardiogram; HQ = hydroxychloroquine; HTN = hypertension; IHD = ischemic heart disease; LBBB = left bundle branch block; MI = myocardial infarction; NSAIDs = nonsteroidal anti-inflammatory drugs; RBBB = right bundle branch block; SHD = structural heart disease; TTE = transthoracic echocardiogram.
Diagnostic Evaluation
If a patient with moderate or higher risk develops symptoms, or if screening identifies abnormalities listed in Table 1, clinicians should have a high index of suspicion for AM cardiotoxicity. Prompt initiation of workup should be pursued with a CV specialist, including TTE, cMRI, and ambulatory cardiac ECG monitoring for detection of potentially dangerous tachyarrhythmias and bradyarrhythmias that require intervention. If workup is concerning for AM cardiotoxicity, drug discontinuation should be discussed promptly with the patient's rheumatologist. In cases of CHF with preserved or reduced ejection fraction, GDMT may be of benefit in improving symptoms and echocardiographic function. Other potential etiologies should be excluded when relevant and, if diagnostic uncertainty remains, EMB should be considered after evaluation of risks and benefits. The recommended diagnostic approach to a patient taking chronic AM therapy with new CV symptoms or abnormal findings on CV screening is detailed in Figure 3.
Figure 3: Antimalarial Cardiotoxicity: Risk-Based Approach to Drug Monitoring and Evaluation in Symptomatic Patients or Patients With New Abnormal Findings on Drug Monitoring ECG/TTE
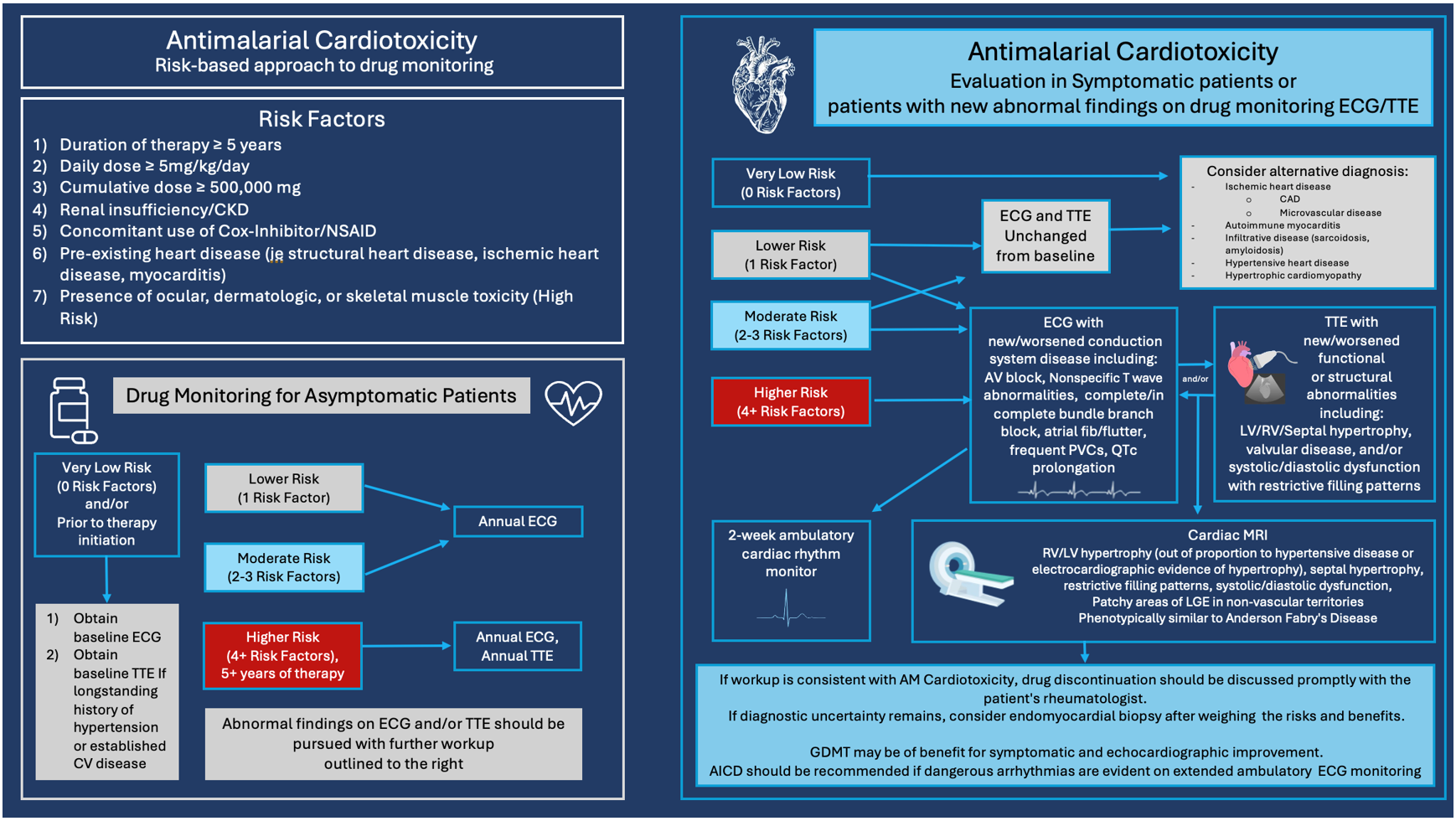
In conclusion, although AM agents exhibit some cardioprotective effects for atherosclerotic disease and related risk factors in patients with underlying autoimmune disease, the risk of AM cardiotoxicity is of major concern for the growing number of patients who are exposed to long-term AM use. Appropriate screening for cardiotoxicity associated with AM therapy is increasingly necessary to prevent the development of this potentially fatal complication. In the absence of data from large randomized studies and strong clinical evidence, these preliminary screening recommendations and workup for AM cardiotoxicity are intended to serve as a potential guide to clinicians until formal screening recommendations can be developed and validated.
References
- Purmah Y, Cornhill A, Lei LY, et al. Mid-wall striae fibrosis predicts heart failure admission, composite heart failure events, and life-threatening arrhythmias in dilated cardiomyopathy. Sci Rep 2022;12:1739.
- Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2018 Jan;77:98-103.
- Kerr G, Aujero M, Richards J, et al. Associations of hydroxychloroquine use with lipid profiles in rheumatoid arthritis: pharmacologic implications. Arthritis Care Res (Hoboken) 2014;66:1619-26.
- Fram G, Wang DD, Malette K, et al. Cardiac complications attributed to hydroxychloroquine: a systematic review of the literature pre-COVID-19. Curr Cardiol Rev 2021;17:319-27.
- Chatre C, Roubille F, Vernhet H, Jorgensen C, Pers YM. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf 2018;41:919-31.
- Desmarais J, Rosenbaum JT, Costenbader KH, et al. American College of Rheumatology white paper on antimalarial cardiac toxicity. Arthritis Rheumatol 2021;73:2151-60.
- Tselios K, Gladman DD, Harvey P, Akhtari S, Su J, Urowitz MB. Abnormal cardiac biomarkers in patients with systemic lupus erythematosus and no prior heart disease: a consequence of antimalarials? J Rheumatol 2019;46:64-9.
- Shalmon T, Thavendiranathan P, Seidman MA, et al. Cardiac magnetic resonance imaging T1 and T2 mapping in systemic lupus erythematosus in relation to antimalarial treatment. J Thorac Imaging 2023;38:W33-W42.
- Yusuf IH, Charbel Issa P, Ahn SJ. Hydroxychloroquine-induced retinal toxicity. Front Pharmacol 2023;14:[ePub ahead of print].
- Cohen IV, Makunts T, Moumedjian T, Issa MA, Abagyan R. Cardiac adverse events associated with chloroquine and hydroxychloroquine exposure in 20 years of drug safety surveillance reports. Sci Rep 2020;10:[ePub ahead of print].
Clinical Topics: Cardio-Oncology, Heart Failure and Cardiomyopathies, Acute Heart Failure, Prevention, Noninvasive Imaging
Keywords: Heart Failure, Cardiotoxicity, Antimalarials, Cardio-oncology, Rheumatology