Beyond Glycemic Control: Sodium-Glucose Cotransporter-2 Inhibitor and Glucagon-Like Peptide-1 Receptor Agonist to Reduce the Burden of Peripheral Artery Disease
Quick Takes
- Sodium-glucose cotransporter-2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP1RA) not only lower blood sugar but also reduce cardiovascular events and improve outcomes in patients with peripheral artery disease (PAD), addressing a critical residual cardiovascular risk.
- Emerging evidence highlights the vascular benefits of GLP1RA and SGLT2i, from reducing revascularization needs (GLP1RA) to improving endothelial function (SGLT2i), positioning them as essential tools in modern PAD management.
- Recent trials suggest that GLP1RA and SGLT2i may not only reduce cardiovascular events but also enhance peripheral perfusion and functional capacity, redefining PAD management in patients with diabetes mellitus.
Over the past decade, lipid-lowering therapies have established the low-density lipoprotein-cholesterol dogma of the lower, the earlier, the longer, the better as the cornerstone of pharmacological prevention for atherosclerotic cardiovascular disease (ASCVD). At the same time, the high recurrence of cardiovascular (CV) events despite optimal medical therapy (OMT) has suggested the concept of residual cardiovascular risk, defined as the presence of CV risk factors not adequately targeted due to poor awareness of their clinical impact or lack of specific drugs. Despite its high global prevalence, peripheral artery disease (PAD) remains frequently underdiagnosed and undertreated, constituting a critical residual CV risk factor and a clinical accelerator of major adverse cardiac events (MACE), major adverse limb events (MALE), and cerebrovascular disease. A post hoc analysis of the FOURIER (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) trial brilliantly emphasized the higher panvascular risk of individuals with PAD: patients with symptomatic PAD experienced a higher rate of any acute arterial event when compared with patients with coronary artery disease or cerebrovascular disease. Moreover, patients with symptomatic PAD exhibited similar rates of acute coronary events to those with previous myocardial infarction as well as comparable rates of acute cerebrovascular events to those with prior stroke.1
In this context, sodium-glucose cotransporter-2 inhibitors (SGLT2i) and glucagon-like peptide-1 receptor agonists (GLP1RA) represent promising glucose-lowering strategies not only to reach optimal glucose control but also to address additional cardiometabolic risk factors associated with PAD. Current guidelines recommend SGLT2i (canagliflozin, dapagliflozin, and empagliflozin) and GLP1RA (liraglutide and semaglutide) in patients with diabetes mellitus (DM) and PAD beyond their glycated hemoglobin or concomitant glucose-lowering therapies since they demonstrated to reduce MACE.2,3
SGLT2i and GLP1RA lower blood glucose through different mechanisms of action: SGLT2i block sodium-glucose cotransporter-2 protein in the proximal renal tubule, which reabsorbs most of the glucose filtered by the glomerulus, thereby inducing glycosuria, natriuresis, and diuresis4; vice versa, GLP1RA mimic the action of endogenous glucagon-like peptide-1 synthesized in intestinal L-cells, pancreatic alpha cells, and neurons in the solitary tract nucleus, and so the activation of GLP1RA enhances insulin secretion and inhibits glucagon release.5 Besides DM, visceral adipose tissue, inflammation, hypertension, and dyslipidemia constitute key risk factors for patients with PAD and therapeutic targets for these new glucose lowering-drugs.
However, the underestimation of PAD as a systemic atherosclerotic and chronic inflammatory disease is highlighted by the fact that, although commonly reported as an adverse event, it has never been evaluated as a primary or secondary endpoint in CV outcome trials except the SUSTAIN-6 (Trial to Evaluate Cardiovascular and Other Long-Term Outcomes With Semaglutide in Subjects With Type 2 Diabetes) where the incidence of revascularization (coronary and peripheral), which was included among the secondary outcomes, was significantly reduced by semaglutide. Further encouraging data come from the SELECT (Semaglutide Effects on Cardiovascular Outcomes in People With Overweight or Obesity) study, in which there was a numerical reduction of vascular disorders among patients without DM and with overweight or obesity who were treated with semaglutide.6 In the future, the results of the STRIDE (A Research Study to Compare a Medicine Called Semaglutide Against Placebo in People With Peripheral Arterial Disease and Type 2 Diabetes) trial will provide data on the effects of semaglutide on functional capacity in patients with DM and symptomatic PAD,7 interesting insights on the peripheral vascular benefit of GLP1RA come from the Italian STARDUST (Effects of the GLP-1 Receptor Agonist Liraglutide on Lower Limb Perfusion in People With Type 2 Diabetes and Peripheral Artery Disease: An Open-Label Randomized Clinical Trial), which found that among individuals with DM and PAD, 6 months subcutaneous liraglutide was associated with a significant increase of peripheral perfusion detected by transcutaneous oxygen pressure compared with OMT.8 Moreover, compared with the control group, the individuals treated with liraglutide achieved a significant improvement of 6-min walking distance, a significant reduction of C-reactive protein, and higher increase in circulating levels of endothelial progenitor cells and vascular endothelial growth factor (VEGF) A. These results are in line with a prespecified secondary analyses of the LYDIA (Effects of Liraglutide in Young Adults With Type 2 DIAbetes) trial in which liraglutide treatment resulted in higher circulating concentrations of VEGF and stromal cell-derived factor-1-alpha compared with sitagliptin. Indeed, benefits of these agents may be substantial even in individuals without DM and with PAD and obesity.
In contrast, since the CANVAS (Canagliflozin Cardiovascular Assessment Study) reported a higher rate of lower extremity amputations in patients with DM treated with canagliflozin, safety concerns were raised about the use of SGLT2i in patients with PAD. Still, this evidence was not confirmed in the CREDENCE (Canagliflozin and Renal Events in Diabetes With Established Nephropathy Clinical Evaluation) trial, which evaluated canagliflozin in patients with DM and chronic kidney disease, and was further averted by a pooled analysis of CANVAS and CREDENCE, which showed no increased risk of MALE with canagliflozin and its greater benefits in patients with PAD. Furthermore, another patient-level pooled analysis of >11,000 individuals from the DAPA-HF (Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction) and DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure) trials provided additional robust evidence regarding SGLT2i safety, demonstrating that while amputations were more frequent in patients with heart failure and PAD compared with those without PAD, the rates of these events were similar between dapagliflozin and placebo-treated patients, irrespective of PAD status. While GLP1RA appears to provide a greater anti-inflammatory vascular benefit, interesting data have emerged on the endothelial effects of SGLT2i. A recent randomized controlled trial assessed the impact of dapagliflozin on the vasomotor function of macrocirculation and microcirculation and found that 12-week treatment of dapagliflozin was associated with significant improvement in brachial flow‑mediated vasodilation (FMD) and nitric oxide production when compared with glibenclamide in patients with DM and subclinical ASCVD.9 The improvement in endothelial function should be considered a class effect of the SGLT2i since it was demonstrated that, among patients with DM, empagliflozin outperformed dapagliflozin in terms of better FMD.10
In conclusion, the multidistrict action of GLP1RA and SGLT2i provide a unique opportunity to simultaneously address multiple pathological pathways driving PAD progression (Figure 1). Their integration into PAD management could significantly improve outcomes and quality of life for patients with DM or without DM who have high atherothrombotic risk.
Figure 1: Therapeutic Targets of SGLT2i and GLP1RA to Reduce the Burden of Peripheral Artery Disease
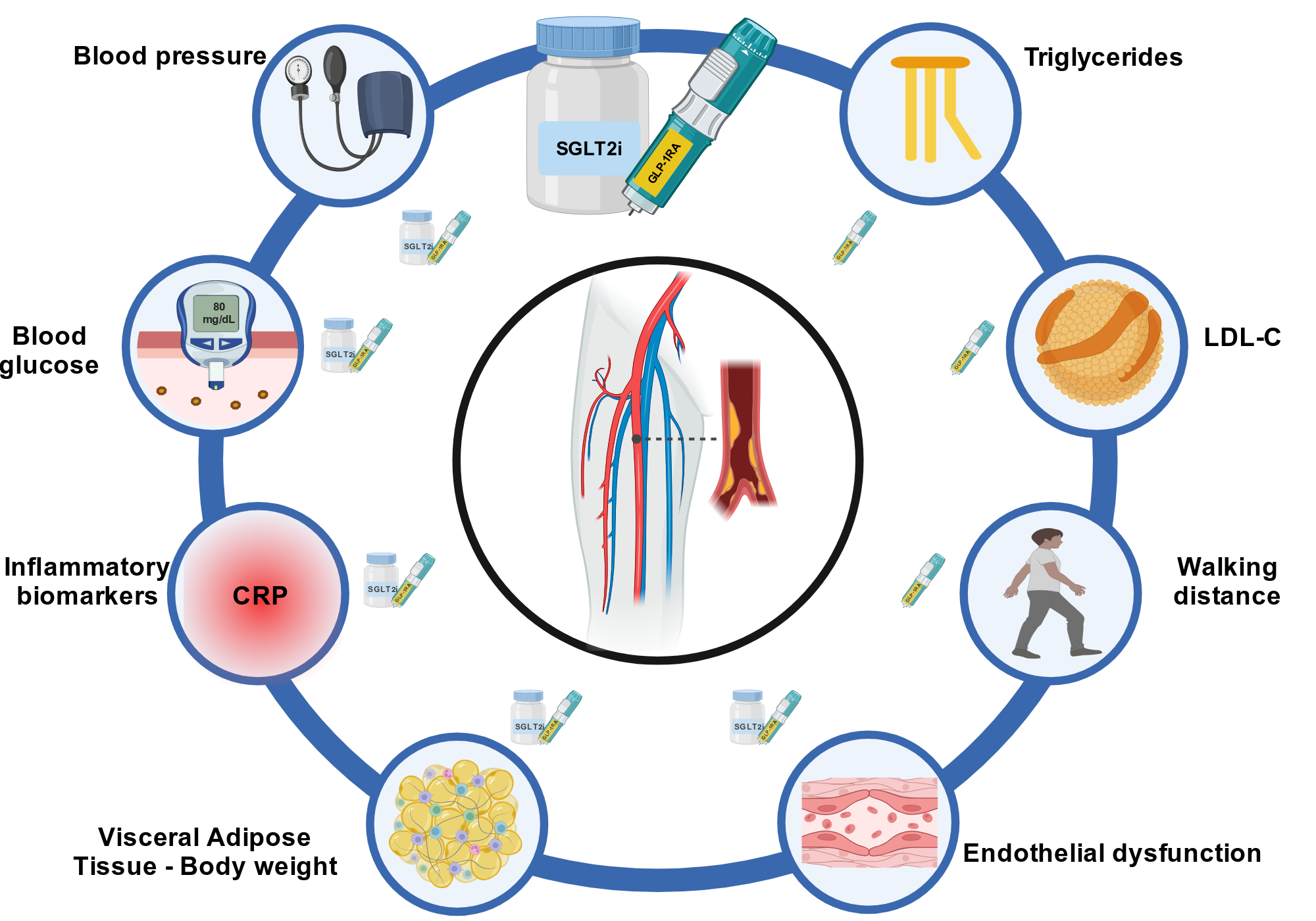
Created in BioRender. Vergallo, R. (2025) https://BioRender.com/p38e372.
CRP = C-reactive protein; GLP1RA = glucagon-like peptide-1 receptor agonist; LDL-C = low-density lipoprotein cholesterol; SGLT2i = sodium-glucose cotransporter-2 inhibitor.
References
- Oyama K, Giugliano RP, Tang M, et al. Effect of evolocumab on acute arterial events across all vascular territories: results from the FOURIER trial. Eur Heart J 2021;42:4821-9.
- Gornik HL, Aronow HD, Goodney PP, et al.; Peer Review Committee Members. 2024 ACC/AHA/AACVPR/APMA/ABC/SCAI/SVM/SVN/SVS/SIR/VESS guideline for the management of lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2024;149:e1313-e1410.
- Mazzolai L, Teixido-Tura G, Lanzi S, et al.; ESC Scientific Document Group. 2024 ESC guidelines for the management of peripheral arterial and aortic diseases. Eur Heart J 2024;45:3538-700.
- Packer M, Wilcox CS, Testani JM. Critical analysis of the effects of SGLT2 inhibitors on renal tubular sodium, water and chloride homeostasis and their role in influencing heart failure outcomes. Circulation 2023;148:354-72.
- Zheng Z, Zong Y, Ma Y, et al. Glucagon-like peptide-1 receptor: mechanisms and advances in therapy. Signal Transduct Target Ther 2024;9:234.
- Lincoff AM, Brown-Frandsen K, Colhoun HM, et al.; SELECT Trial Investigators. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med 2023;389:2221-32.
- Bonaca MP, Catarig AM, Hansen Y, et al. Design and baseline characteristics of the STRIDE trial: evaluating semaglutide in people with symptomatic peripheral artery disease and type 2 diabetes. Eur Heart J Cardiovasc Pharmacother 2025;10:728-37.
- Caruso P, Maiorino MI, Longo M, et al. Liraglutide for lower limb perfusion in people with type 2 diabetes and peripheral artery disease. JAMA Netw Open 2024;7:[ePub ahead of print].
- Sposito AC, Breder I, Soares AAS, et al.; ADDENDA-BHS2 trial investigators. Dapagliflozin effect on endothelial dysfunction in diabetic patients with atherosclerotic disease: a randomized active-controlled trial. Cardiovasc Diabetol 2021;20:74.
- Balleza Alejandri LR, Grover Páez F, González Campos E, et al. Empagliflozin and dapagliflozin improve endothelial function in Mexican patients with type 2 diabetes mellitus: a double-blind clinical trial. J Cardiovasc Dev Dis 2024;11:182.
Clinical Topics: Vascular Medicine, Atherosclerotic Disease (CAD/PAD), Diabetes and Cardiometabolic Disease
Keywords: Peripheral Arterial Disease, Glucagon-Like Peptide 1, Sodium-Glucose Transporter 2 Inhibitors