Emerging Gene Therapies For Familial Hypercholesterolemia
Background
Given advancements in the understanding of the genetic underpinnings of several cardiovascular diseases (CVDs), there is an increased focus on the use of targeted gene therapies in the treatment of some of these conditions.1,2 To this end, both the American Heart Association (AHA) and the European Society of Cardiology (ESC) have published articles discussing gene therapy.1,2 This article summarizes the AHA and ESC gene therapy reviews and elaborates on gene therapy in the management of familial hypercholesterolemia (FH).
Gene therapies can be broadly categorized into two types based on their mechanism of action: 1) agents that either target RNA, typically to silence gene activity; or 2) agents that directly edit DNA (Figure 1).1,2
Figure 1: Mechanisms for Gene Therapy in Familial Hypercholesterolemia
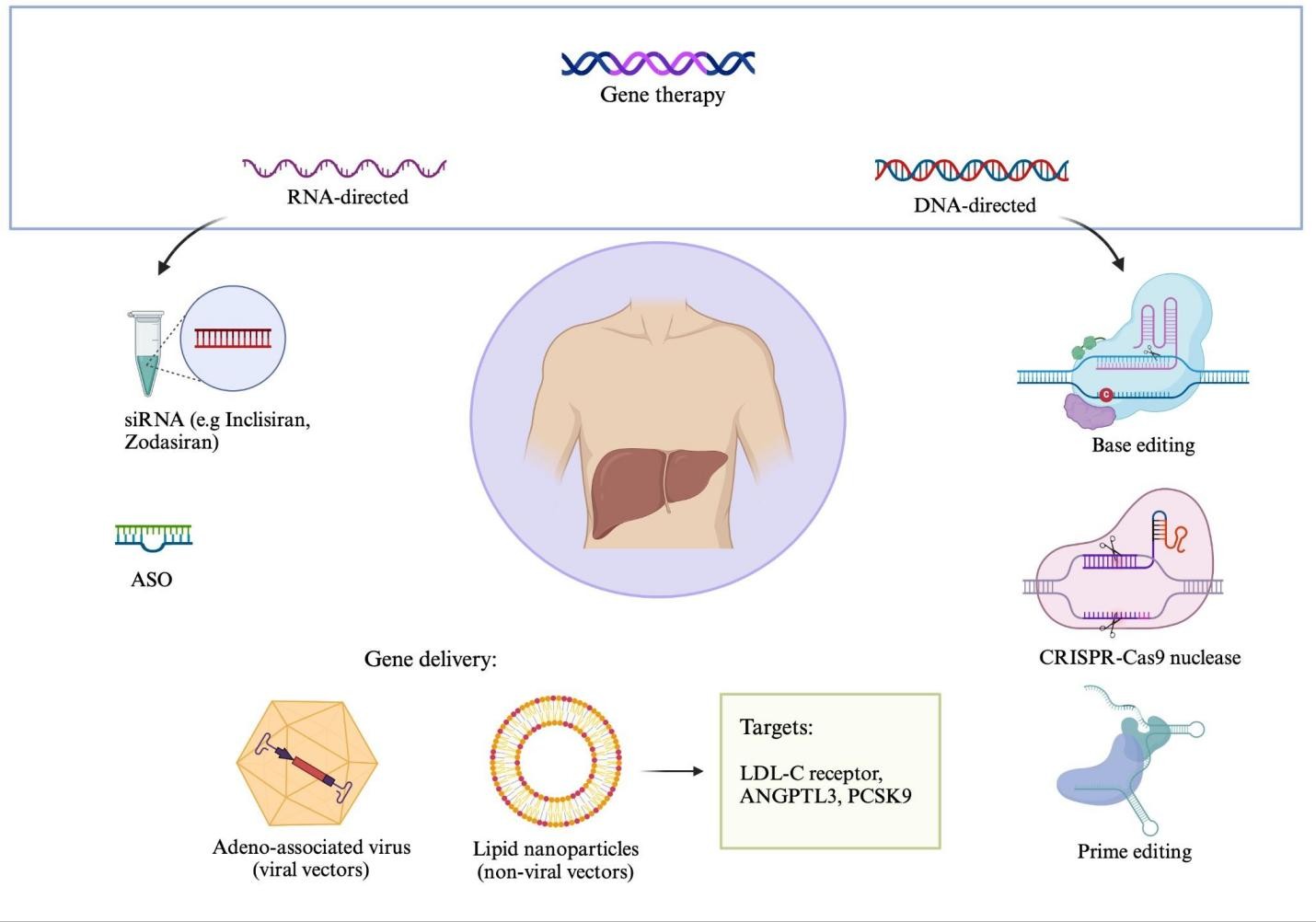
Created in BioRender. Ugoala O. https://BioRender.com/l5g29id.
ANGPTL3 = angiopoietin-like protein 3; ASO = antisense oligonucleotide; LDL-C = low-density lipoprotein cholesterol; PCSK9 = proprotein convertase subtilisin/kexin type 9; siRNA = small interfering RNA.
Strategies to target RNA include small interfering RNA (siRNA) or antisense oligonucleotides (ASOs), whereas gene therapies that alter DNA typically use CRISPR-Cas9 nucleases, base editors, or prime editors.1,2 In order to target the gene therapy agent to the appropriate tissue, a delivery mechanism is often necessary. Viral vectors such as adeno-associated virus or nonviral vectors such as lipid nanoparticles may be used for this purpose.1,2 Overall, there have been further advancements in the agents used in targeting RNA, with some being approved for clinical use, compared with DNA-editing therapies.
The appropriate type of gene therapy used depends on the genetics of the relevant disease.1,2 Conditions associated with the loss of, or insufficient normal gene function, may be rescued by directly editing the pathogenic DNA sequence or replacement of the gene.1,2 In contrast, conditions associated with production of harmful proteins may be rescued by directly editing the pathogenic variant or silencing the related RNA product.1,2 Monogenic CVD, which results from pathogenic variants at a single genetic locus such as FH, are optimal targets for gene therapy.1,2
FH is an inherited condition that causes elevation in low-density lipoprotein cholesterol (LDL-C) and is associated with an increased risk of atherosclerotic CVD (ASCVD).3,4 FH is most commonly associated with pathogenic variants in the LDL-C receptor gene, but may also be due to pathogenic variants in apolipoprotein B (apoB) or proprotein convertase subtilisin/kexin type 9 (PCSK9).3,4 There are two main types of FH: 1) heterozygous FH (HeFH), in which one allele at one locus has a pathogenic variant; and 2) homozygous FH (HoFH), in which both alleles at a locus have a pathogenic variant or two loci have pathogenic variants.3,4 Studies have shown that HoFH is associated with earlier onset of disease and worse outcomes than HeFH.3,4
Management of Familial Hypercholesterolemia
Conventional treatment of FH centers on the reduction of LDL-C to reduce ASCVD risk.4,5 The initial treatment goal is the reduction of LDL-C by ≥50%, with consideration of target LDL-C <100, <70, or <55 mg/dL depending on ASCVD risk.5 Statins are typically first-line pharmacologic therapy and may be combined with ezetimibe, a PCSK9 inhibitor, a bile acid sequestrant, or bempedoic acid.4,5 Statins and PCSK9 inhibitors may be less efficacious in HoFH due to significantly decreased LDL-C receptor activity.4 In that case, other treatment strategies may include lipid apheresis but this is expensive, invasive, and time-consuming.4,5 Lomitapide, a small molecule inhibitor of microsomal triglyceride transfer protein, or evinacumab, a monoclonal antibody that inhibits angiopoietin-like protein 3 (ANGPTL3), are also possible pharmacotherapies.4,5
Gene Therapy for Familial Hypercholesterolemia
Multiple RNA-targeting agents have been investigated for the treatment of FH. Mipomersen and vupanorsen are both ASOs directed toward hepatocytes targeting apoB100 and ANGPTL3, respectively.4 They were initially approved by the Food and Drug Administration (FDA) for the treatment of FH but their approval was withdrawn due to hepatic adverse effects.4 A phase I clinical trial investigating the use of an ASO targeting PCSK9 was completed (NCT03427710), although the results are not yet available.4 Inclisiran is an siRNA approved by the FDA for the treatment of HeFH or ASCVD in cases in which LDL-C is not at goal.4,6 It targets PCSK9 and is delivered as a subcutaneous injection twice per year.4,6 The ORION-9 (Inclisiran for Heterozygous Familial Hypercholesterolemia–9) trial demonstrated inclisiran's efficacy in reducing LDL-C in patients with HeFH, whereas the ORION-5 (A Study of Inclisiran in Participants With Homozygous Familial Hypercholesterolemia–5) trial demonstrated that inclisiran is not effective in HoFH.7 ARO-ANG3 (zodasiran) is an siRNA that targets ANGPTL3 and is currently in a phase II trial (NCT05217667) with interim positive results reported, after a successful phase I trial.2,4,8
Agents that directly alter the genome are also in development. For example, AAV8-guided LDL-C transgene directed to the liver has shown promising preclinical studies with the completion of the first human study (NCT02651675) and is currently undergoing 5 years of follow-up (NCT04080050).1,4 Another AAV8-guided LDL-C receptor for HoFH study is ongoing in China (NCT06125847).1 Some base editors are currently being studied with the intention of treating HoFH or HeFH. VERVE-201 is one of such base editors targeting ANGPTL3, currently in preclinical studies, and is intended to treat HoFH.2 VERVE-101 is a base editor targeting PCSK9 and is intended to treat HeFH.2,4,9 However, the phase Ib clinical trial for VERVE-101 was stopped due to liver enzyme elevations and thrombocytopenia.2,4,9 VERVE-102 is a similar agent with a different lipid nanoparticle delivery system that is now in phase 1b trials (NCT06164730).2,4,9
Conclusion
Gene therapy offers an important opportunity to address inherited CVD such as FH. Considering the immediate clinical impact of these therapies, RNA-targeting agents, such as siRNA and ASOs, are the furthest in development currently, with FDA-approved agents like inclisiran available. These agents carry fewer risks associated with mutagenesis or off-target genomic effects compared with direct DNA agents.1,2,4 However, they still carry risks of organ toxicity, as demonstrated by mipomersen and vupanorsen.1,2,4 RNA-targeting agents also avoid ethical questions associated with direct genome editing.1,2,4 Gene editing or replacement techniques remain appealing because they hold the possibility of decreasing reliance on regular medications and provide a chance to fully cure the condition.1
The cost associated with these treatments will likely remain high, significantly limiting access.1,2 For example, Casgevy, the first FDA-approved CRISPR-based treatment, offers a cure for sickle cell disease but was released at a price of $2.2 million per patient.10 Additionally, access to appropriate FH treatment is predicated on clinical evaluation and in some cases access to genetic testing necessary to diagnose FH, which may be limited by cost and other systemic barriers.1,2 Thus, gene therapy agents currently in development offer exciting possibilities for the treatment of FH, but many require significantly more research before clinical application, and access to these treatments may be limited by cost and other systemic barriers.
References
- Kim Y, Landstrom AP, Shah SH, Wu JC, Seidman CE; American Heart Association. Gene therapy in cardiovascular disease: recent advances and future directions in science: a science advisory from the American Heart Association. Circulation. 2024;150(23):e471-e480. doi:10.1161/CIR.0000000000001296
- Dimmeler S, Ferri L, Nioi P, et al. Translation of genomics into routine cardiological practice: insights from a European Society of Cardiology Cardiovascular Round Table. Eur Heart J. 2025;46(15):1384-1393. doi:10.1093/eurheartj/ehaf041
- Parsamanesh N, Kooshkaki O, Siami H, Santos RD, Jamialahmadi T, Sahebkar A. Gene and cell therapy approaches for familial hypercholesterolemia: an update. Drug Discov Today. 2023;28(3):103470. doi:10.1016/j.drudis.2022.103470
- Damase TR, Sukhovershin R, Godin B, Nasir K, Cooke JP. Established and emerging nucleic acid therapies for familial hypercholesterolemia. Circulation. 2024;150(9):724-735. doi:10.1161/CIRCULATIONAHA.123.067957
- Watts GF, Gidding SS, Hegele RA, et al. International Atherosclerosis Society guidance for implementing best practice in the care of familial hypercholesterolaemia. Nat Rev Cardiol. 2023;20(12):845-869. doi:10.1038/s41569-023-00892-0
- Gaine SP, Quispe R, Patel J, Michos ED. New strategies for lowering low density lipoprotein cholesterol for cardiovascular disease prevention. Curr Cardiovasc Risk Rep. 2022;16(9):69-78. doi:10.1007/s12170-022-00694-y
- Raal F, Durst R, Bi R, et al. Efficacy, safety, and tolerability of inclisiran in patients with homozygous familial hypercholesterolemia: results from the ORION-5 Randomized Clinical Trial. Circulation. 2024;149(5):354-362. doi:10.1161/CIRCULATIONAHA.122.063460
- Watts GF, Schwabe C, Scott R, et al. RNA interference targeting ANGPTL3 for triglyceride and cholesterol lowering: phase 1 basket trial cohorts. Nat Med. 2023;29(9):2216-2223. doi:10.1038/s41591-023-02494-2
- Hooper AJ, Tang XL, Burnett JR. VERVE-101, a CRISPR base-editing therapy designed to permanently inactivate hepatic PCSK9 and reduce LDL-cholesterol. Expert Opin Investig Drugs. 2024;33(8):753-756. doi:10.1080/13543784.2024.2369747
- Rueda J, de Miguel Beriain Í, Montoliu L. Affordable pricing of CRISPR treatments is a pressing ethical imperative. CRISPR J. 2024;7(5):220-226. doi:10.1089/crispr.2024.0042
Clinical Topics: Dyslipidemia, Homozygous Familial Hypercholesterolemia, Prevention
Keywords: Hypercholesterolemia, Genetic Therapy