Contrast Media in Advanced Cardiovascular Imaging: Clinical Questions and Shifting Paradigms
Quick Takes
- For patients with stable estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2, the risk of kidney injury from iodinated contrast material is low; however, the risk is uncertain in those with eGFR <30 mL/min/1.73 m2, in those with acute kidney injury, and in those without anuria who are receiving dialysis.
- Patients with a prior hypersensitivity reaction to the same class of contrast media (iodine or gadolinium based) are at increased risk of a subsequent hypersensitivity reaction, and this risk can be mitigated by switching agents; iodine-containing substances (e.g., shellfish, topical povidone-iodine) have no cross reactivity with iodinated contrast material.
- Group II gadolinium-based contrast agents have minimal risk (0-0.07%) of nephrogenic systemic fibrosis even in patients at higher risk (eGFR <30 mL/min/1.73 m2) with standard doses (≤0.1 mmol/kg); recent consensus statements have deemed kidney function screening optional.
Intravenous Iodinated Contrast Use in Cardiac Computed Tomography Angiography
Contrast-Associated Acute Kidney Injury
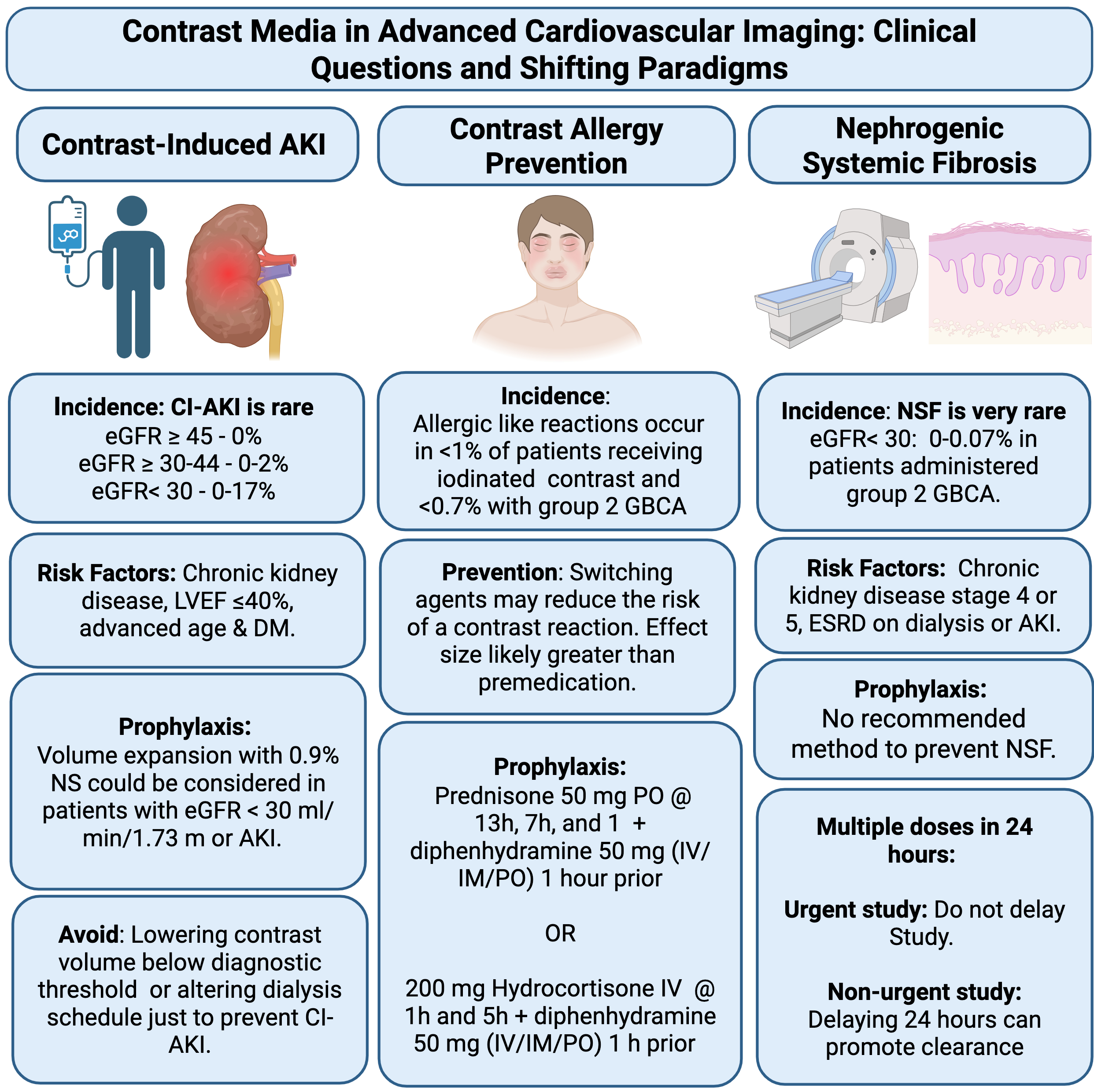
The 2021 multisociety Guideline for the Evaluation and Diagnosis of Chest Pain elevated coronary computed tomography angiography (CTA) as a key diagnostic tool for evaluating symptomatic patients without prior coronary artery disease. Additionally, the use of cardiac CTA has expanded significantly in areas such as pulmonary vein ablation and structural intervention planning.1 However, contrast-associated acute kidney injury (CA-AKI), defined as acute kidney injury (AKI) of any cause (i.e., not specific to the contrast material) occurring within 48 hours after intravenous (IV) contrast material administration, remains a common concern. A subset of CA-AKI is contrast-induced acute kidney injury (CI-AKI; i.e., AKI caused by contrast material). Fear of CI-AKI often prompts the selection of alternative, less optimal imaging modalities, potentially delaying diagnosis and prolonging hospital stays.2 The risk of CA-AKI is increased in patients with pre-existing chronic kidney disease, systolic dysfunction, advanced age, diabetes mellitus, sepsis, and many other conditions.2 Historical studies suggest a CA-AKI incidence of approximately 10% in patients with estimated glomerular filtration rate (eGFR) 45-59 mL/min/1.73 m2, and of up to 30% in patients with eGFR <30 mL/min/1.73 m2. However, contemporary studies suggest that estimates of CI-AKI (in contradistinction to CA-AKI) have been substantially overestimated due to selection bias and the lack of a comparable control group. In more recent studies using advanced statistical methods to address confounding, the incidence of CI-AKI has been estimated to be 0% in patients with eGFR ≥45 mL/min/1.73 m2, 0-2% in patients with eGFR 30-44 mL/min/1.73 m2, and 0-17% in patients with eGFR <30 mL/min/1.73 m2 (Figure 1).2,3 Thus, for patients with stable eGFR ≥30 mL/min/1.73 m2, the risk of CI-AKI is very low, but for those with glomerular filtration rate (GFR) <30 mL/min/1.73 m2, those with AKI, or those without anuria who are receiving dialysis, the risk is unclear.3 This uncertainty makes IV contrast use in these populations a relative contraindication.
Figure 1: Contrast Media in Advanced Cardiovascular Imaging: Clinical Questions and Shifting Paradigms
Created in BioRender. Bhatia, K. (2025) https://BioRender.com/m3o5aay
AKI = acute kidney injury; CI-AKI = contrast-induced acute kidney injury; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; ESRD = end-stage renal disease; GBCA = gadolinium-based contrast agents; IM = intramuscular; IV = intravenous; LVEF = left ventricular ejection fraction; NS = normal saline; NSF = nephrogenic systemic fibrosis.
Imaging decisions in patients at potential risk (i.e., eGFR <30 mL/min/1.73 m2, AKI, those without anuria who are receiving dialysis) should be individualized and alternative modalities should be considered. When alternatives are possibly inferior, such as in planning for transcatheter aortic valve implantation, in which CTA-guided valve sizing results in larger implant size and reduces postprocedural aortic regurgitation compared with a transesophageal echocardiography–guided approach, contrast-enhanced imaging with computed tomography may still be preferred.4 In such scenarios, the potential risk of CA-AKI should be explained to the patient prior to the study. Guidelines suggest prophylactic volume expansion with 0.9% normal saline as a measure to partially mitigate the risk of CA-AKI in patients at high risk.3 However, the results of a recent randomized trial in patients with eGFR 30-59 mL/min/1.73 m2 showed similar rates of AKI and increased risk of heart failure with saline administration.5
Risk Reduction in Patients With Prior Hypersensitivity Reaction to Contrast Material
Iodinated Contrast Agents
Allergic-like reactions to IV nonionic iodinated contrast media occur in <1% of cases, with severe reactions in 0.04%. Patients with a prior hypersensitivity reaction to the same group of contrast media are at increased risk of a subsequent hypersensitivity reaction. This risk can be mitigated by switching agents. Although premedication with corticosteroids and antihistamines is common, especially in the United States, the evidence supporting that practice is weak. Iodine-containing substances (e.g., shellfish, topical povidone-iodine) have no cross reactivity with iodinated contrast material and are not a contraindication. When given, the most common prophylaxis regimen includes 50 mg of oral prednisone administered 13 hours, 7 hours, and 1 hour before contrast administration, along with an antihistamine 1 hour prior.3 The effectiveness of this regimen in preventing severe allergic-like reactions is limited and breakthrough reactions can still occur. For patients with a prior allergic reaction to contrast media who are not premedicated or for inpatients, an accelerated IV steroid regimen is noninferior to the standard oral regimen. This involves administering two doses of methylprednisolone 40 mg IV or hydrocortisone 200 mg IV, given 5 hours and 1 hour prior to contrast administration. This regimen allows for a shorter wait time and reduces overall length of stay. Alternatively, in patients with a known reaction to a specific contrast formulation, substituting the agent can be more effective in preventing repeat allergic-like reactions than premedication.3
Gadolinium-Based Contrast Agents
Serious allergic reactions to gadolinium-based contrast agents (GBCAs) are rare (0.004-0.7% of case), with life-threatening anaphylaxis being extremely rare (0.001-0.01%).6 The frequency of adverse reactions is approximately eight times higher in patients with a previous reaction to a GBCA. The ACR® Manual on Contrast Agents suggests that it may be reasonable to premedicate or consider a different agent in patients with a prior reaction to a GBCA who need contrast-enhanced magnetic resonance imaging (MRI); however there are no published studies to establish the efficacy of this recommendation.6
GBCA Use in Cardiovascular MRI
The use of cardiac MRI has steadily increased due to expanded indications and improved accessibility. Cardiac MRI using GBCAs is the reference standard for evaluation of myocardial fibrosis in multiple cardiac pathologies. There is confusion among referring providers regarding which patients can safely receive GBCAs, but the landscape has changed with the increased use of group II (macrocyclic) agents.
Nephrogenic Systemic Fibrosis
Nephrogenic systemic fibrosis (NSF) is a very rare but potentially fatal multisystem disorder linked to GBCA use, primarily affecting the skin and subcutaneous tissues in patients with advanced kidney disease (GFR <30 mL/min/1.73 m2).7 In 2007, the Food and Drug Administration (FDA) issued a black box warning to avoid the use of GBCAs in patients with acute or chronic kidney insufficiency (GFR <30 mL/min/1.73 m2). NSF risk varies among GBCA types—nearly all unconfounded cases are linked to group I agents (primarily linear), whereas group II agent (macrocyclic and gadobenate dimeglumine) administration is associated with little to no risk.6 The change in FDA labeling coupled with preferential use of lower-risk group II GBCAs has led to a significant decline in NSF cases.6 A 2020 meta-analysis of 16 studies found a risk of 0-0.07% in patients with eGFR <30 mL/min/1.73 m2 receiving a group II agent.8 These findings informed the 2021 American College of Radiology/National Kidney Foundation (ACR/NKF) consensus statements on the Use of IV Gadolinium-Based Contrast Media in Patients With Kidney Disease, which made kidney function screening optional with standard doses (≤0.1 mmol/kg) of these agents.7 Despite these strong safety data, approximately 20% of centers still refrain from offering contrast-enhanced cardiac MRI to patients with eGFR <30 mL/min/1.73 m2. No effective prophylaxis exists to prevent NSF, and current consensus advises that dialysis should not be initiated or adjusted solely to prevent NSF with standard group II doses (Figure 1). If multiple contrast-enhanced MRIs are urgently needed, subsequent doses of group II contrast agents should not be delayed for fear of NSF; however, if the studies are not urgent, delaying subsequent doses by 24 hours or performing dialysis in between studies can promote GBCA clearance.7
Gadolinium Retention
Findings of recent studies have demonstrated retention of gadolinium months to years after administration in multiple organs including bone, brain, skin, kidney, liver, and spleen.7 Retention has been shown to be the lowest for macrocyclic GBCAs. To date, adverse clinical consequences of gadolinium retention have not been established. However, minimizing repetitive GBCA imaging studies is recommended, particularly studies that are closely spaced in time.9
References
- Writing Committee Members, Gulati M, Levy PD, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines [published correction appears in J Am Coll Cardiol. 2024 Oct 29;84(18):1771. doi: 10.1016/j.jacc.2024.09.024.]. J Am Coll Cardiol. 2021;78(22):e187-e285. doi:10.1016/j.jacc.2021.07.053
- Davenport MS, Perazella MA, Nallamothu BK. Contrast-induced acute kidney injury and cardiovascular imaging: danger or distraction?. Circulation. 2023;147(11):847-849. doi:10.1161/CIRCULATIONAHA.122.062783
- Davenport MS, Perazella MA, Yee J, et al. Use of intravenous iodinated contrast media in patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology. 2020;294(3):660-668. doi:10.1148/radiol.2019192094
- Hayashida K, Bouvier E, Lefèvre T, et al. Impact of CT-guided valve sizing on post-procedural aortic regurgitation in transcatheter aortic valve implantation. EuroIntervention. 2012;8(5):546-555. doi:10.4244/EIJV8I5A85
- Nijssen EC, Rennenberg RJ, Nelemans PJ, et al. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, open-label, non-inferiority trial. Lancet. 2017;389(10076):1312-1322. doi:10.1016/S0140-6736(17)30057-0
- ACR Committee on Drugs and Contrast Media. ACR® Manual on Contrast Media (American College of Radiology website). 2025. Available at: https://www.acr.org/clinical-resources/clinical-tools-and-reference/contrast-manual. Accessed 07/21/2025.
- Weinreb JC, Rodby RA, Yee J, et al. Use of intravenous gadolinium-based contrast media in patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation. Radiology. 2021;298(1):28-35. doi:10.1148/radiol.2020202903
- Woolen SA, Shankar PR, Gagnier JJ, MacEachern MP, Singer L, Davenport MS. Risk of nephrogenic systemic fibrosis in patients with stage 4 or 5 chronic kidney disease receiving a group II gadolinium-based contrast agent: a systematic review and meta-analysis. JAMA Intern Med. 2020;180(2):223-230. doi:10.1001/jamainternmed.2019.5284
- U.S. Food & Drug Administration. FDA Drug Safety Communication: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warnings (FDA website). 2018. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-gadolinium-based-contrast-agents-gbcas-are-retained-body. Accessed 07/21/2025.
Clinical Topics: Cardiovascular Care Team, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Interventions and Imaging, Computed Tomography, Nuclear Imaging
Keywords: Contrast Media, Acute Kidney Injury, Computed Tomography Angiography
