PURSUIT: Once-Daily Novel Oral PCSK9 Inhibitor Effective For Hypercholesterolemia
AZD0780, a novel, once-daily oral molecule inhibitor, had a robust, dose-dependent effect in lowering LDL-C and a favorable safety profile in patients with hypercholesterolemia already on background moderate- or high-intensity statin treatment, according to research presented during a Featured Clinical Research session at ACC.25 in Chicago and simultaneously published in JACC.
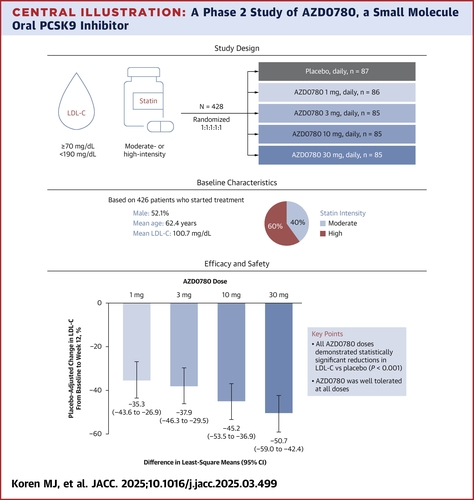
The double-blind, phase 2 PURSUIT study, conducted at 55 research sites in North America, Europe and Asia, randomized 428 patients to either receive oral AZDO780 at 1, 3, 10 or 30 mg once daily or placebo for 12 weeks. The patients were 62 years old on average, half were men and most (86%) were White. Their mean LDL-C level was 100.7 mg/dL.
Of the patients 40% were taking a moderate-intensity statin and 60% a high-intensity statin therapy, and 20% were also taking ezetimibe.
Results showed a dose-dependent reduction in the primary endpoint of percent change in LDL-C from baseline to week 20. The placebo-corrected difference in the least squares mean percent change were –35%, –38%, –45% and –51% for the 1, 3, 10 and 30 mg doses, respectively, compared to placebo.
Use of moderate- vs. high-intensity statin therapy did not seem to affect outcome. LDL-C reductions began one week into treatment and reached a steady-state at two weeks for all treatment groups.
Adverse events were comparable between the treatment and placebo arms (38.2% vs. 32.6%). Twelve patients (3.5%) in the treatment arms and three (3.5%) in the placebo group had adverse events considered possibly related to treatment. The most common adverse event was hypertension; no deaths occurred.
"The development of a well-tolerated, daily pill that could reliably lower LDL-C to a patient's target range would offer a substantial therapeutic advance," wrote study authors Michael J. Koren, MD, FACC, et al., noting that "limited access and the inconvenience of obtaining and using injectable drugs as second-line agents," is a major treatment barrier.
Study limitations included low enrollment of Black patients and the exclusion of patients over 75 years of age or with elevated triglycerides or high-sensitivity C-reactive protein.
Keywords: ACC Annual Scientific Session, ACC25, Homozygous Familial Hypercholesterolemia
< Back to Listings
