Evolut Low Risk: TAVR Noninferior to SAVR at 5-Year Follow-Up
Patients with severe aortic stenosis who were treated with either TAVR or surgery had comparable rates of all-cause mortality or disabling stroke at five years, according to research presented during a Late-Breaking Clinical Trial session at ACC.25 in Chicago and simultaneously published in JACC. Valve durability and performance were also excellent in both groups.
In the international Evolut Low Risk trial, investigators randomized 1,414 patients (mean age 74 years, 35% women) with severe aortic stenosis at low surgical risk to either Evolut TAVR (n=730) or SAVR (n=684).
At five years, data were available on 91.9% and 87.4% of the patients in the TAVR and SAVR groups. Of these patients, the primary endpoint of death or disabling stroke occurred in 15.5% of those receiving Evolut TAVR and 16.4% of those undergoing SAVR (hazard ratio [HR], 0.90; p=0.47). In the respective groups, the rate of all-cause mortality was 13.5% and 14.9%; disabling stroke 3.6% and 4.0%; cardiovascular mortality 7.2% and 9.3%; and noncardiovascular mortality was 6.8% and 6.2%.
After a vital status sweep of patients lost to follow-up or withdrew from the study, all-cause mortality was 14.7% and 15.2% of the TAVR and SAVR groups (HR, 0.96; p=0.74).
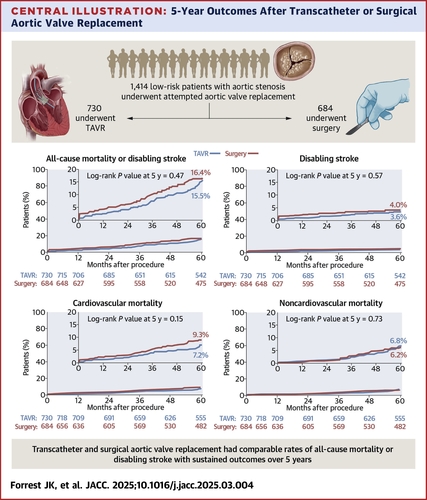
Hemodynamics were better with TAVR, with a significantly lower mean aortic valve gradient (10.7 mm Hg vs. 12.8 mm Hg with SAVR) and the mean effective orifice area was significantly larger (2.1 cm2 vs. 1.9 cm2). In the SAVR group, there was a lower rate of new pacemaker placement and less mild paravalvular regurgitation.
Both arms had comparable rates of valve reintervention (3.3% for TAVR vs. 2.5% for SAVR; p=0.44) and quality of life (KCSS score of 88.3 for TAVR vs. 88.5 for SAVR), as well as rates of aortic valve rehospitalization, endocarditis, myocardial infarction, reintervention and valve thrombosis.
"The outcomes of all-cause mortality and disabling stroke remain similar between TAVR and surgery, though numerically TAVR remains better," said Michael J. Reardon, MD, FACC, the study's senior author. "TAVR has also shown similar improvements in terms of symptom and functional class and there's no difference in the rate of reinterventions, so the supra-annular, self-expanding Evolut TAVR looks really good at five years in this group of patients."
In an accompanying editorial comment, Tsuyoshi Kaneko, MD, FACC, et al., note the increasing popularity of TAVR, with more than 100,000 TAVRs being performed in 2023 vs. less than 60,000 SAVRs, noting that a past criticism was lack of long-term outcomes in younger patients with longer life expectancy.
"Although the 5-year result of this trial provides compelling evidence with no significant negative signal against TAVR in terms of the main clinical outcomes and valve performance, our journey to understanding the lifetime management of aortic stenosis is still at its dawn," write the authors. "It is an exciting time in our field to navigate aortic valve interventions, especially in younger low-risk patients, and we will further await the long-term outcome of Evolut Low-Risk and PARTNER 3 because both trials are scheduled for a 10-year follow-up."
Clinical Topics: Cardiac Surgery, Invasive Cardiovascular Angiography and Intervention
Keywords: ACC Annual Scientific Session, ACC25, Transcatheter Aortic Valve Replacement, Interventional Cardiology, Stenosis
< Back to Listings
