FINEARTS-HF: Finerenone Improves Outcomes Regardless of Baseline Kidney Risk
Finerenone was found to consistently improve clinical outcomes as well as heart failure (HF)-related health status and albuminuria, in patients with HF with mildly reduced ejection fraction (HFmrEF) or HF with preserved ejection fraction (HFpEF) regardless of baseline kidney risk, according to an analysis from the FINEARTS-HF trial published in JACC: Heart Failure.
In the prospective, double-blind trial, 6,001 participants with chronic HFmrEF or HFpEF were randomized to either placebo or a target dose of 20 or 40 mg of finerenone based on an eGFR of ≤60 or >60 mL/min/1.73m2. Patients with an eGFR of <25 mL/min/1.73m2 or serum potassium >5.0 mmol/L were excluded.
At baseline, most patients (5,797) fit one of three Kidney Disease: Improving Global Outcomes (KDIGO) risk score categories, with 35% categorized as low risk (eGFR ≥60 mL/min/1.73m2 and urine albumin-to-creatinine ratio [UACR] <30 mg/g); 29% as moderate risk (either eGFR ≥60 mL/min/1.73m2 and UACR ≥30 to ≤300 mg/g or eGFR ≥45 to 59 mL/min/1.73m2 and UACR <30 mg/g); and 36% as high or very high risk (eGFR ≥45 to <60 mL/min/1.73m2 and UACR ≥30 to 300 mg/g; eGFR <45 mL/min/1.73m2; or UACR >300 mg/g). Participants with higher kidney risk tended to be older, women, self-reported as Asian, and have worse HF-related health status, history of hospitalization and more comorbidities.
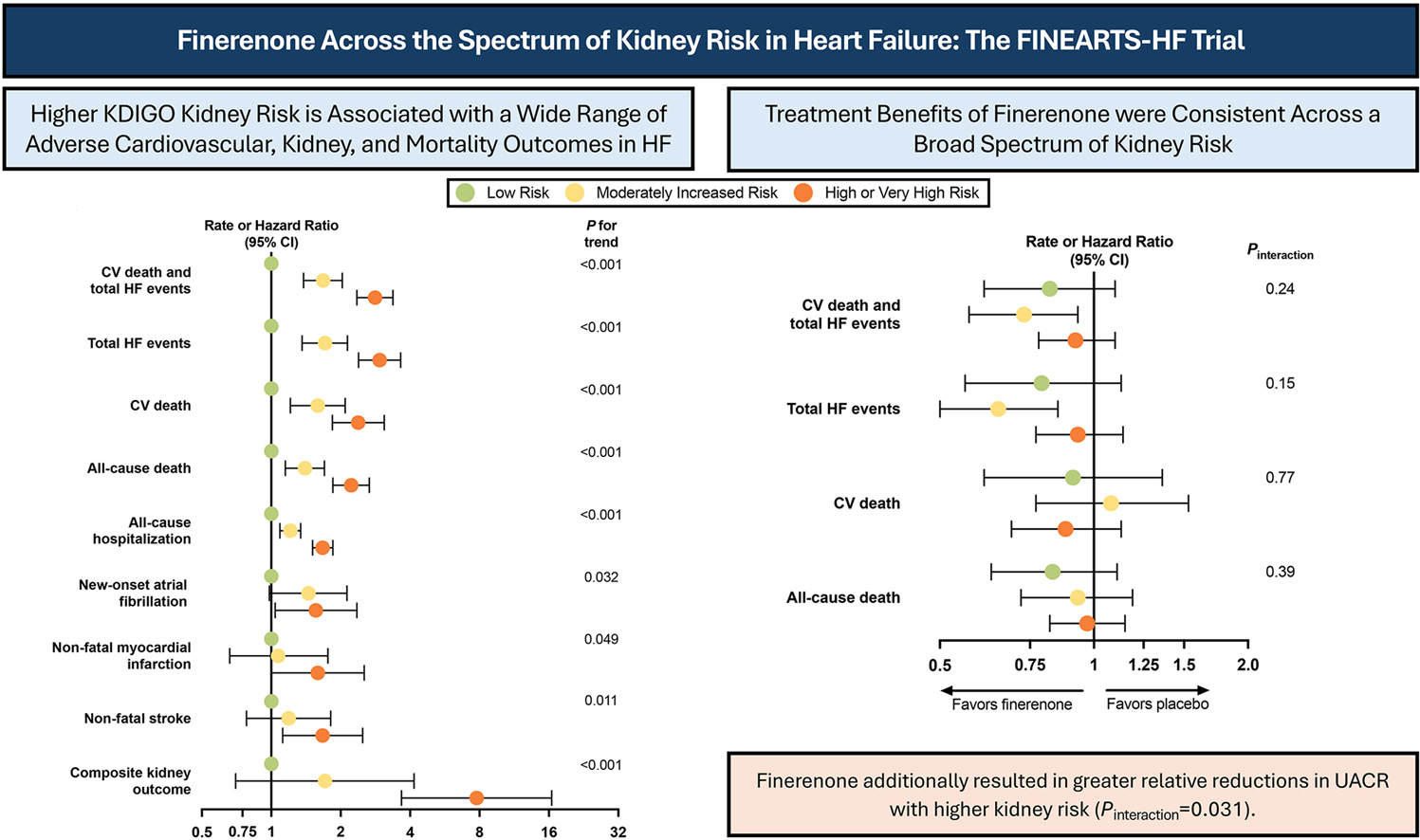
Results showed that at 12 months, regardless of the KDIGO category, finerenone reduced the combined primary endpoint of cardiovascular death and total HF events (pint=0.24), as well as improved HF-related health status as assessed by the Kansas City Cardiomyopathy Questionnaire-Total Symptom Score (pint=0.36). In addition, there was no difference in safety events, including hyperkalemia, in patients with a higher kidney risk.
Over the median 2.7 years of follow-up, a higher baseline kidney risk was associated with a higher rate of the primary outcome and other key endpoints, including the composite kidney outcome, new-onset atrial fibrillation and vascular events, including a seven-fold increase in higher covariate-adjusted rate of composite kidney outcome in the high/very high risk group compared with the low risk group.
However, a higher baseline kidney risk also led to greater absolute treatment benefit with finerenone. The absolute rate reduction was 4.4 per 100-person years in the moderate risk group and 2.1 per 100 person-years in the high/very risk group compared to 1.5 per 100 person-years in the low-risk group.
Participants in the treatment arm with higher kidney risk also experienced a greater reduction in UACR at six months, with a 24% placebo-adjusted mean reduction in the low risk group, 29% in the moderate risk group and 36% in the high/very high risk group (pint=0.031) with no differences in the eGFR slope.
"Further data are needed to determine whether greater reductions in UACR among those with higher kidney risk translates to greater long-term cardiovascular and kidney benefit with finerenone," write John Ostrominski, MD, et al.
They add, "These findings underscore the interconnected pathophysiological pathways linking cardiovascular and kidney disease, and support the use of finerenone to improve clinical outcomes across a wide spectrum of kidney disease severity in HFmrEF/HFpEF."
Clinical Topics: Heart Failure and Cardiomyopathies, Acute Heart Failure
Keywords: Renal Insufficiency, Chronic, Heart Failure, Mineralocorticoid Receptor Antagonists, Albuminuria
< Back to Listings
