CATHEDRAL-HF: Is Carvedilol Effective as Monotherapy in HFimpEF?
Withdrawal of other heart failure treatment while maintaining carvedilol as a single therapy did not worsen left ventricular (LV) function compared to usual treatment at 52 weeks in patients with heart failure with improved ejection fraction (HFimpEF), according to a brief report on the CATHEDRAL-HF trial published in JACC: Heart Failure.
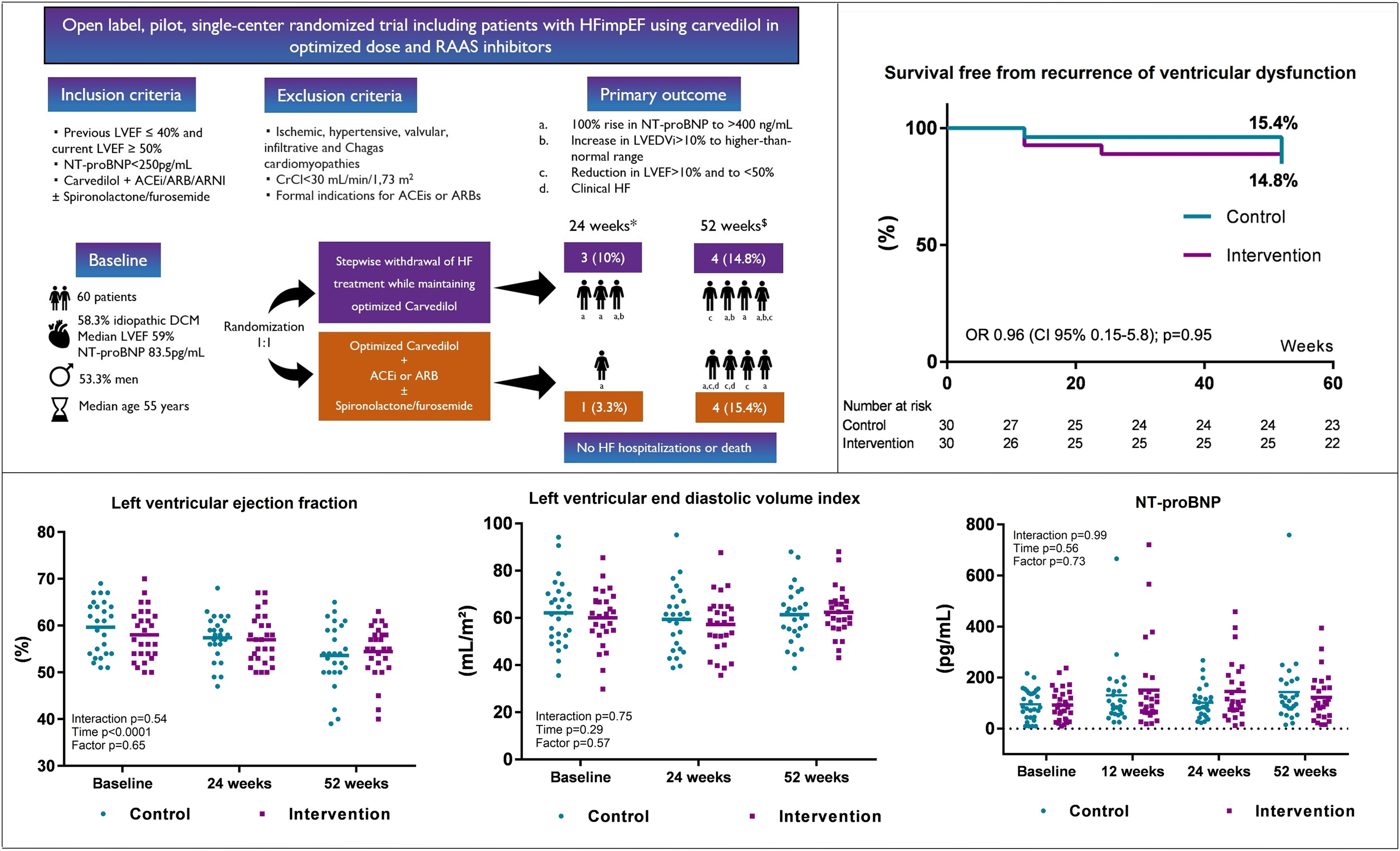
In the open-label, pilot, single-center clinical trial conducted in Brazil, Deborah de Sá Pereira Belfort, MD, et al., randomized 60 patients (median age 55 years; 53% men) with HFimpEF (previous LVEF ≤40% and current LVEF ≥50%) 1:1 to either a control group following usual treatment for HF with reduced ejection fraction (HFrEF) or an intervention group with a stepwise reduction in doses of medication every two weeks until suspension of all therapies except carvedilol.
The daily dose of carvedilol was increased before randomization if the dose was <50 mg/d and if heart rate was >60 beats/min. In the intervention group, spironolactone and/or furosemide were withdrawn first, followed by ACE inhibitors, ARBs or angiotensin receptor neprilysin inhibitors (ARNIs).
Patients were eligible for participation if they had no HF symptoms, an NT-proBNP level <250 pg/mL and were using maximum tolerated doses of carvedilol (median daily dose of 50 mg) plus an ACE inhibitor, ARB or ARNI, with or without spironolactone and/or furosemide.
Idiopathic dilated cardiomyopathy was the most common HF etiology (58%) among patients. Median LVEF was 59%, median LV global longitudinal strain was –15.5% and median NT-proBNP was 83.5 pg/mL. In total, 70% of patients took spironolactone. All were on optimal doses of either enalapril or losartan, none took an ARNI and one patient was taking an SGLT2 inhibitor.
Results showed that at 24 weeks, one patient in the control group (3.3%) and three in the intervention group (10%) experienced the primary endpoint – recurrence of ventricular dysfunction, defined as one or more of the following: absolute reduction in LVEF by >10% to <50%; increase in LV end diastolic volume index (LVEDVi) to body surface area by >10% and to the higher-than-normal range; 100% increase in NT-proBNP to >400 ng/mL; and/or clinical evidence of HF.
At 52 weeks, four patients in the control group (15.4%) and four in the intervention group (14.8%) experienced a recurrence of ventricular dysfunction, and one patient in the control group (3.8%) experienced atrial flutter. There were no hospitalizations, deaths or sustained ventricular arrhythmias in either group throughout the follow-up period; LVEF, LVEDVi and NT-proBNP remained similar between the two groups.
"This is the first study to show the feasibility of discontinuing HF therapy while maintaining carvedilol in HFimpEF," write the authors. "We believe this trial provides valuable insights for further studies to confirm these findings and to assess different pharmacological strategies maintaining beta-blockers in patients with HFimpEF."
Clinical Topics: Heart Failure and Cardiomyopathies, Statins, Acute Heart Failure
Keywords: Carvedilol, Heart Failure, Adrenergic beta-Antagonists
< Back to Listings
