Advancements in Pacemaker Technology: The New Leadless Device
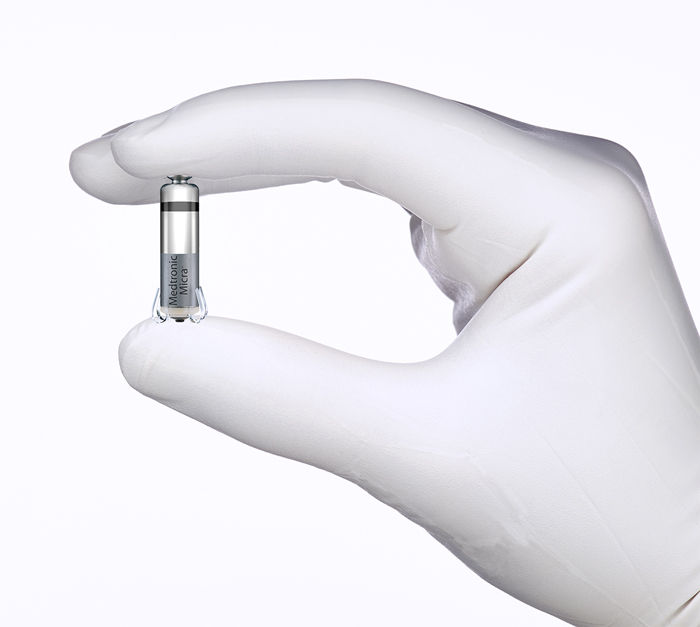
On April 6, the U.S. Food and Drug Administration (FDA) approved the first leadless, transcatheter pacemaker system. The Micra Transcatheter Pacing System from Medtronic, works like other pacemakers to regulate heart rate but is designed to avoid the need for a pacemaker pocket and transvenous lead. The Micra device is 93 percent smaller than current pacemaker systems, takes up less than 1 percent volume of the right ventricle, is approximately one-inch-long and weighs only 2.0 grams.
The device is a single-chamber system, pacing only the right ventricle (VVI mode) and is fully self-contained, which eliminates the need for leads, which have always been the weak link in the traditional pacing system. Removing the lead component of the pacing system allows the patient to avoid related lead complications such as fractures, insulation breaches, venous thrombus obstructions and leads across the tricuspid valve which eliminates lead-induced tricuspid regurgitation. This system is ideal for patients with prior cardiovascular implantable electronic device infection and venous access issues. Micra is also magnetic resonance imaging- (MRI-) safe and is FDA approved for 1.5 and 3.0 Tesla scans.
In November 2015, results from the the Micra multicenter clinical trial were published in the New England Journal of Medicine. The trial involved 56 centers in 19 countries, enrolled 744 patients and evaluated the safety and efficacy of the device. More than 99 percent of patients in the trial were successfully implanted (719 of 725 patients). After six months 98 percent of patients implanted had low and stable pacing capture thresholds, and 96 percent met the primary safety endpoint. There were no dislodgements or infections.
The implant procedure takes a total of 30 – 40 minutes. It is performed through a single femoral venous access, using a 23 French diameter introducer sheath. A delivery catheter is fed from the access site up through the inferior vena cava, into the right atrium, passing down through the tricuspid valve, with implantation in the apex of the right ventricle. Linear one-step deployment of four tines that are attached to the device allows for fixation to the right ventricle and for optimal electrode to tissue interaction, decreasing thresholds and prolonging battery life (estimated 10 – 12 year battery longevity). During implant, the device can be repositioned and recaptured, if necessary. Once final placement is achieved and the device is released, pacing begins, and the accelerometer is able to adjust heart rate to pace the patient based on their activity level. The catheter is removed, and a single suture closure is placed at the access site. Same day discharge is possible. The venous access implant site has no bump or scar, and there are no post-implant activity restrictions. It completely eliminates pocket-related complications such as infections, hematoma, and erosion. The cell becomes encapsulated by tissue over time, and by battery end-of-life, a new device would be placed in close proximity to the existing one.
The system has a convenient off switch that allows for upgrade of treatment needs or discontinuation of pacing if no longer needed. The device was designed to allow patients to be followed by their physician and send data remotely via the Medtronic CareLink programmer, and is expected to be available later this year.
The leadless pacemaker is indicated for patients with a class I or II indication for pacing based on guidelines: patients diagnosed with atrial fibrillation (AFib)with complete atrioventricular (AV) block or bradycardia, post AV nodal ablation and pacing for AFib , intermittent sinus arrest, or procedural AV block with the possibility of conduction recovery (post valve replacement), or in symptomatic paroxysmal or permanent high grade AV block with no AFib, as an alternative to dual-chamber pacing when atrial lead placement is considered difficult, high risk, or not necessary for the effective therapy.
Because the system is approved for VVI pacing only, the device is contraindicated in patients with pure sinus node dysfunction, patients with an implanted inferior vena cava filter, a mechanical tricuspid valve, or in patients with an implanted cardiac device providing other cardiac therapy that may interfere with the sensing performance of the Micra device. Other contraindications include femoral venous anatomy unable to accommodate the delivery of the device, the inability for a patient to accommodate a 23 French introducer sheath or difficulty with implants on the right side of the heart due to obstruction or severe tortuosity, and morbid obesity that prevents telemetry communication from the device. It is also contraindicated for patients with a known intolerance to heparin, sensitivity to contrast media, and those that cannot tolerate a single dose of 1.0 mg dexamethasone acetate.
This revolutionary device provides the opportunity for safe and effective treatment in patients needing VVI pacing, with a great reduction in the complications commonly associated with traditional pacing systems.
This article was authored by Mary Leier, MScN, FNP-BC, a nurse practitioner for the Electrophysiology and Heart Rhythm Center at Cedars Sinai Heart Institute, Cedars-Sinai Medical Center in Los Angeles, CA.