A 61-year-old female patient presented to the emergency department of the National Institute of Cardiology Ignacio Chavez with dyspnea of moderate effort, oppressive chest pain, and weight loss of 18 kg over the last 2 months. At admission, she presented dyspnea at rest. The electrocardiogram showed inversion of the T wave in V1-V4 and S1, Q3 and T3 pattern, suggesting pulmonary embolism (PE), but pulmonary scintigraphy and angiotomography ruled out the diagnosis of PE. The laboratory showed elevated troponin I of 0.209 ng/ml (normal range 0.0-0.04) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) of 2,202 pg/ml (normal range 100-400), and the diagnosis of acute coronary ischemic syndrome without ST-segment elevation was established; therefore, antianginal and antithrombotic treatment was initiated. Transthoracic echocardiography reported dilatation of the right cavities, moderate tricuspid regurgitation, severe pulmonary hypertension with systolic pulmonary artery pressure of 107 mmHg, borderline right ventricular systolic function (tricuspid annular plane systolic excursion of 16 mm), and normal left ventricular systolic function (left ventricular ejection fraction of 55%) with impaired relaxation pattern (Figure 1).
Figure 1
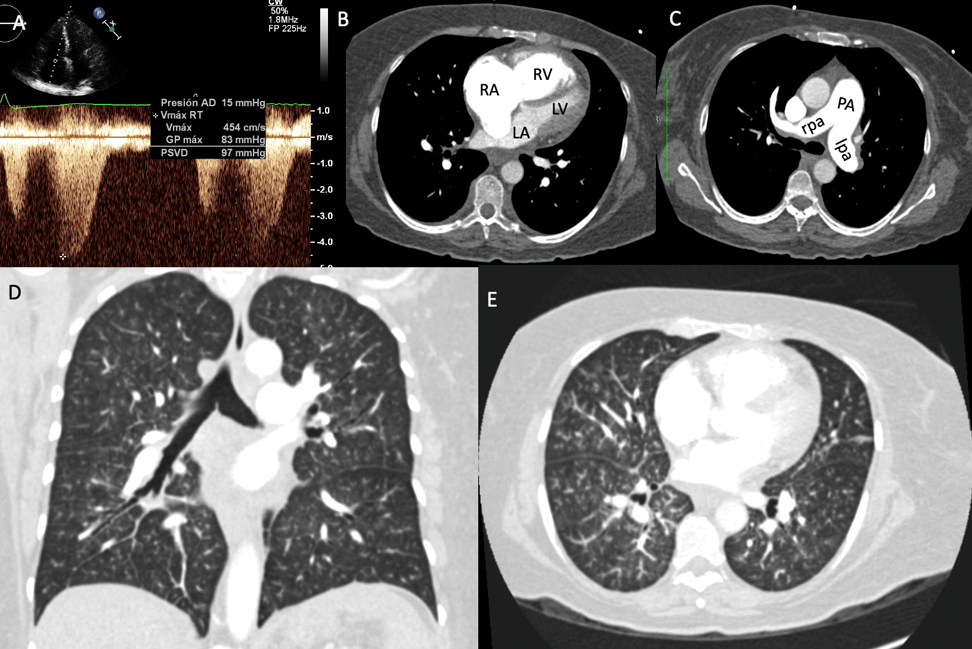 (A) Systolic pulmonary artery pressure by echocardiogram. (B-C) Dilation of right cavities, pulmonary artery, and pulmonary branches. (D-E) Angiotomography of the lung without evidence of PE or fibrosis.
(A) Systolic pulmonary artery pressure by echocardiogram. (B-C) Dilation of right cavities, pulmonary artery, and pulmonary branches. (D-E) Angiotomography of the lung without evidence of PE or fibrosis.
Spirometry showed mild restriction:
- FEV1(L) 1.41 prebronchodilator and 1.56 postbronchodilator
- FEV1/FVC (%) 81 prebronchodilator and 82 postbronchodilator
- FVC (L) 1.74 prebronchodilator and 1.90 postbronchodilator
The patient was evaluated by the Cardioneumology Department due to poor prognosis criteria for ischemic heart disease (angina and elevation of NT-proBNP and troponin I), cough, and hyaline expectoration. The diagnosis of pulmonary hypertension of probable idiopathic etiology was established, and treatment with acenocumarine (oral anticoagulant), furosemide, spironolactone, and sildenafil was started. There was clinical improvement, and she was discharged home. The cardiac catheterization performed 2 weeks later reported right atrium pressure of 3 mmHg, systolic right ventricle pressure of 84 mmHg and mean pressure of 34 mmHg, systolic and mean pulmonary artery pressures of 81 mmHg and 49 mmHg, respectively, and capillary pulmonary pressure of 11 mmHg. Cardiac output was 8.04 L/min with cardiac index of 5.04 and negative vasoreactivity test.
A month later, she presented again with dizziness, nausea, malaise, dyspnea on rest, and diarrhea. The arterial blood gas showed mixed alkalosis. The electrocardiogram showed no changes in relation to the previous one, and the PE was ruled out again by angiotomography. At this admission, she was anxious, pale, dehydrated, tachycardic (heart rate of 116 bpm), and tachypneic (respiratory rate of 28 rpm) with decreased oxygen levels (SpO2 of 74% at FiO2 of 21%) that improved with the administration of oxygen (SpO2 of 80%). The NT-proBNP was 8,428 pg/ml. The patient had a torpid evolution, and on the second day of her hospitalization, she developed acute renal injury and remained hypoxemic with SpO2 of 74%, despite the administration of oxygen. She received treatment with dobutamine, dopamine, digoxin, nitric oxide, and bosentan with clinical and laboratorial improvement. Two days later, she presented suddenly with hemodynamic deterioration. A week later, she died due to cardiogenic shock and severe pulmonary hypertension.
The correct answer is: D. Pulmonary hypertension with unclear and/or multifactorial mechanisms
This patient's condition first presented with precordial pain, evidence of right heart failure, and a great weight loss in a short time. Laboratory, echocardiography, angiotomography, spirometry, and cardiac catheterization studies allowed us to rule out the presence of ischemic heart disease, PE, and pulmonary fibrosis.
In the pathological study, hypertrophy of the right ventricular free wall was detected. The histological study of the lung showed multiple neoformation cells, which occupied the peribronchial lymphatic vessels. Abundant fibrosis was observed in the arterioles, with complete obstruction of the lumen and proliferation of the intima that corresponds to mature angiomatoid or plexiform lesion consistent with primary pulmonary arterial hypertension.1 The bronchial tree had normal characteristics, without evidence of primary neoplasia in situ. Primary neoplasia in the liver, spleen, kidney, adrenal glands, small bowel, colon, and thyroid gland was ruled out. The markers estrogen, progesterone, and HER 2 were compatible with primary breast cancer (Figure 2).1-5
Figure 2
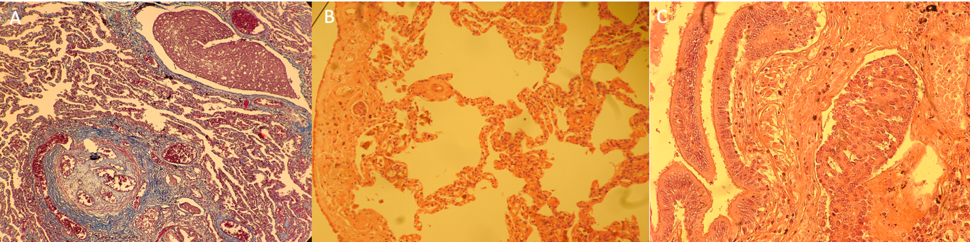 (A) Lung photomicrograph showing an occluded pulmonary arteriole corresponding to a mature angiomatoid or plexiform lesion. In addition, a dilated lymphatic vessel by a sheet of ovoid neoplastic epithelial cells and monotones of red color are observed. (B) Mesothelium with neoplastic cells. (C) No histopathological alterations in the terminal bronchiole. Lymphatic vessel with ovoid neoplastic epithelial cells.
(A) Lung photomicrograph showing an occluded pulmonary arteriole corresponding to a mature angiomatoid or plexiform lesion. In addition, a dilated lymphatic vessel by a sheet of ovoid neoplastic epithelial cells and monotones of red color are observed. (B) Mesothelium with neoplastic cells. (C) No histopathological alterations in the terminal bronchiole. Lymphatic vessel with ovoid neoplastic epithelial cells.
Pulmonary tumor emboli can produce occlusion of pulmonary microvasculature and subacute and progressive picture of thromboembolic disease. Microscopic tumor embolism is rarely recognized before death due to the difficulty in establishing the diagnosis.2-5
Based on the diagnostic studies and histopathology, we conclude that the patient had a diagnosis of multifactorial pulmonary arterial hypertension, according to the clinical classification of pulmonary hypertension guidelines.1
References
- Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119.
- Vincent F, Lamblin N, Classe M, et al. Subacute right heart failure revealing three simultaneous causes of post-embolic pulmonary hypertension in metastatic dissemination of breast cancer. ESC Heart Failure 2017;4:75-7.
- Watanabe H, Ichihara E, Kano H, Ninomiya K, Tanimoto M, Kiura K. Congestive Heart Failure During Osimertinib Treatment for Epidermal Growth Factor Receptor (EGFR)-mutant Non-small Cell Lung Cancer (NSCLC). Intern Med 2017;56:2195-7.
- Chen CF, Lin MH, Chu KA, Liu WS, Hsiao SH, Lai RS. Effective cardiac radiotherapy relieved life-threatening heart failure caused by advanced small cell lung cancer with cardiac metastasis: a case report. J Thorac Dis 2018;10:E250-E254.
- Venton G, Turcanu M, Colle J, et al. Pulmonary hypertension in patients with myeloproliferative neoplasms: A large cohort of 183 patients. Eur J Intern Med 2019;68:71-5.


