A Clinical Trial on Trial: The Results of PALLAS
Amiodarone is the most effective pharmacologic agent for the maintenance of sinus rhythm in patients with atrial fibrillation but has substantial organ toxicity which limits its use. To reduce side effects, dronedarone was designed with an absence of iodine in the molecule and with a shorter half-life. Although it has similar electrophysiologic properties as amiodarone, dronedarone is less effective for the prevention of atrial fibrillation. However unlike other antiarrhythmic drugs, it has been shown to reduce important clinical events in patients with atrial fibrillation. In A Placebo Controlled, Double-Blind Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg bid for the Prevention of Cardiovascular Hospitalization or Death from any Cause in PatiENts with Atrial fibrillation/atrial flutter (ATHENA), dronedarone reduced the risk of a combination of first cardiovascular hospitalization and death (Figure 1) in patients with paroxysmal or persistent atrial fibrillation with additional risk factors for death.1 The favorable results were uniformly consistent in pre specified sub groups including patients with structural heart disease, patients with a history of congestive heart failure, and patients with a low ejection fraction. The latter points were important because of a report of excess mortality with dronedarone when used in patients with severe heart failure.2 Based on ATHENA dronedarone was approved for use in the United States to reduce the risk of hospitalization for atrial fibrillation in patients in sinus rhythm who have a history of either paroxysmal or persistent atrial fibrillation.
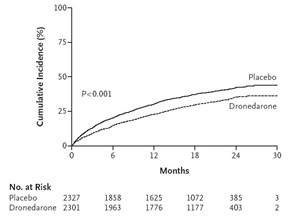 |
| Figure 1. Cumulative incidence of a composite of first hospitalization due to cardiovascular events or death in ATHENA (NEJM 2009; 360:668-78) |
PALLAS was a randomized double blind trial conducted of 489 centers and 37 countries and compared dronedarone with placebo in patients with permanent atrial fibrillation. There were two co-primary outcomes: 1) a composite of stroke, myocardial infarction, systemic embolism or cardiovascular death, and 2) unplanned hospitalization for cardiovascular causes or death. Most of the PALLAS enrolling sites identified a reasonable number of patients with permanent atrial fibrillation. Since dronedarone was not an investigational product and had side effects known to the cardiology community, recruitment was expected to be rapid. In fact, recruitment was more difficult than expected. Many investigators commented that patients with permanent atrial fibrillation felt reasonably well and did not want to take part in a trial involving an additional medication. In addition, during the recruitment phase reports of acute hepatocellular damage including liver failure requiring transplantation were noted in post marketing analyses. This prompted a review of liver function abnormalities in short term placebo controlled trials involving dronedarone in patients with atrial fibrillation. Liver function abnormalities occurred but not more frequently than placebo. Clinicians were advised of concerns about hepatocellular damage, but in the United States periodic monitoring of liver function tests was not required and the trial continued. It was noted that study medications were poorly tolerated. After a median follow up of 3.5 years 13.1% of dronedarone patients had an adverse event leading to treatment discontinuation compared with 5% of patients treated with placebo. Major reasons for discontinuation included diarrhea, asthenia or fatigue, dyspnea or edema, liver function abnormalities, or electrocardiographic changes including prolongation of the QT interval.
PALLAS was terminated prematurely after the enrollment of 3236 patients, well short of the planned 10,800 patients, because of safety concerns and an increase in the first co-primary outcome (Figure 2). There were also statistically significant increases in death of any cause, death from cardiovascular causes, death from cardiac arrhythmia, stroke, unplanned hospitalization for cardiovascular causes, hospitalization for heart failure and heart failure episodes of hospitalization.4
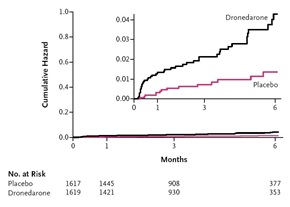 |
| Figure 2. Risk of Stroke, Myocardial Infarction, Systemic Embolism, or Cardiovascular Death in PALLAS (NEJM 2011; 365:2268-76) |
The mechanisms whereby dronedarone worsened outcomes in PALLAS are likely multifactorial. Since over one third of patients in PALLAS were on digoxin and since dronedarone increases digoxin levels, digoxin toxicity was considered to be one explanation and is being explored in more detail. Dronedarone may worsen left ventricular function. The corrected QT interval increased by 8 msec in the dronedarone group and ventricular pro-arrhythmia is a consideration. The excess number of strokes remains unexplained. Most of the patients in PALLAS were on either a vitamin K antagonist or dabigatran. There is a small interaction between dronedarone and vitamin K antagonists, usually with elevation of the INR. Dronedarone inhibits the p-glycoprotein pathway and may result in increased levels of dabigatran. Consequently, under anticoagulation due to a drug interaction between dronedarone and anticoagulants seems less likely as a cause for the increased number of strokes.
The reaction by the cardiovascular community to PALLAS has been polarizing. Some have wondered if the drug should be removed from the market. Other physicians have observed benefit in patients and think use should be restricted to less ill patients. Currently dronedarone is contraindicated in patients with symptomatic heart failure with recent decompensation requiring hospitalization or NYHA Class IV heart failure. It is also contraindicated in patients in atrial fibrillation who cannot or will not be cardioverted into sinus rhythm (permanent atrial fibrillation). Other contraindications include second or third degree heart block or sick sinus syndrome without a pacemaker, bradycardia < 50 bpm, liver toxicity related to previous amiodarone use, severe hepatic impairment, a Qtc Bazett interval > 500 ms or PR > 280 ms, and in patients with concomitant use of strong CYP 3A inhibitors or drugs or herbal preparations that prolong the QT interval and increase the risk of Torsade de Pointes. Women who are pregnant or nursing mothers should not receive dronedarone.
There are important safety precautions when using drondarone. Patients who receive dronedarone should undergo monitoring the cardiac rhythm no less often than every 3 months. The drug should be initiated in patients who are in sinus rhythm and who are receiving appropriate antithrombotic therapy. Maintaining normal potassium and magnesium levels is recommended. A small increase in serum creatinine is observed after initiation of dronedarone due to inhibition of tubular secretion of dronedarone. Periodic and serum creatinine is recommended. If digoxin is used, the dose should be cut in half or monitored carefully. The drug should not be given with other antiarrhythmic drugs. It should be stopped if symptoms of heart failure develop.
We continue to search for safe and effective antiarrhythmic drugs to prevent atrial fibrillation. The search will continue.
References
- Hohnloser S, Crijns H, van Eickels M, Gaudin C, Page R, Torp-Pedersen C, et al. Effect of Dronedarone on Cardiovascular Events in Atrial Fibrillation. N Engl J Med 2009; 360:668-78.
- Køber L, Torp-Pedersen C, McMurray J, Gøtzsche O, Lévy S, Crijns H, et al. Increased Mortality after Dronedarone Therapy for Severe Heart Failure. N Engl J Med 2008; 358:2678-87.
- Nieuwlaat R, Hohnloser SH, Connolly SJ. Effect of Dronedarone in Patients with Permanent Atrial Fibrillation during the ATHENA Study. Eur Heart J 2011; 32:Suppl:618.
- Connolly S, Camm A, Halperin J, Joyner C, Alings M, Amerena J, et al. Dronedarone in High-Risk Permanent Atrial Fibrillation. N Engl J Med 2011; 365:2268-76.
- Flaker GC, Blackshear JL, McBride R, Kronmal RA, Halperin JL, Hart RG. Antiarrhythmic Drug Therapy and Cardiac Mortality in Atrial Fibrillation. J Am Coll Cardiolol 1992; 20:527-32.
Clinical Topics: Arrhythmias and Clinical EP, Dyslipidemia, Heart Failure and Cardiomyopathies, Atherosclerotic Disease (CAD/PAD), Implantable Devices, EP Basic Science, Atrial Fibrillation/Supraventricular Arrhythmias, Lipid Metabolism, Acute Heart Failure
Keywords: Adrenergic Antagonists, Asthenia, Atrial Fibrillation, Coronary Artery Disease, Diarrhea, Digoxin, Dyspnea, Heart Failure, Liver Failure, Myocardial Infarction, ATP Binding Cassette Transporter, Subfamily B, Member 1, Sick Sinus Syndrome, Stroke, United States
< Back to Listings

