Postural Tachycardia Syndrome (POTS) Diagnosis and Treatment: Basics and New Developments
Editor's Note: Please note this author will address off-label use in the following article.
Diagnostic Criteria & Common Clinical Features of POTS
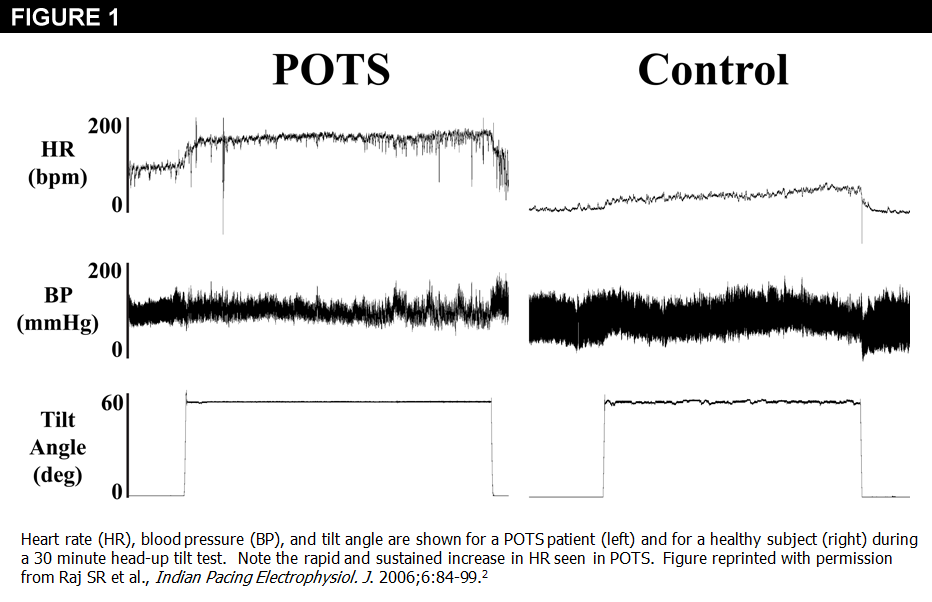
POTS is defined as the presence of chronic symptoms of orthostatic intolerance (≥6 months) accompanied by an increased heart rate (HR) ≥30 bpm within 10 minutes of assuming an upright posture (Figure 1) and in the absence of orthostatic hypotension (blood pressure [BP] fall >20/10 mmHg).1,2 In children and adolescents, a higher threshold (≥40 bpm) should be used since they have a greater physiological orthostatic tachycardia.3 Morning postural assessments will optimize diagnostic sensitivity (at the expense of specificity) for POTS.4 The orthostatic tachycardia must occur in the absence of other overt causes of orthostatic tachycardia (e.g., acute blood loss), medications that impair autonomic regulation, or other chronic debilitating disorders that might cause tachycardia (e.g., anemia, diabetes with known autonomic neuropathy, systemic infectious or inflammatory conditions, hyperthyroidism).
Both cardiac symptoms (rapid palpitation, lightheadedness, dyspnea and chest discomfort) and non-cardiac symptoms (headache [often migraines], tremulousness, nausea, sleep difficulties,5 mental clouding [probably due to diminished attention and not necessarily memory problems[,6 exercise intolerance and chronic fatigue)5 are often present. Activities of daily living, such as bathing or housework, may greatly exacerbate symptoms and resultant fatigue. The chest pain syndrome is rarely associated with epicardial coronary artery obstruction, but may be associated with inferior lead electrocardiographic changes, particularly when upright.7 While pre-syncope and lightheadedness are universal in these patients, only a minority of patients experience frank syncope.
The overwhelming majority of patients with POTS are women (80-85%) of child-bearing age (13-50 years).8,9 Of note, orthostatic tolerance is also reduced in healthy females10, which varies with the menstrual cycle,11 especially in patients with POTS.12 Patients frequently report that their symptoms began following acute stressors (e.g., presumed viral illness, major surgery, injury, or pregnancy) but symptoms may also develop more insidiously. Acutely, patients are often confined to bed for a variable period of time which can rapidly cause hypovolemia and cardiac atrophy of ~ 1%/week,13,14 the physiological consequences of bedrest induced orthostatic intolerance,15 similar to what is observed in astronauts after spaceflight.16 Even 20 hours of bedrest deconditioning may cause upright tachycardia and orthostatic intolerance in previously vigorously active individuals,17 which may lead to a "downward spiral" of orthostatic intolerance, and further bedrest deconditioning. Ultimately, regardless of the precipitating cause, in the chronic state, the physiology of "cardiovascular deconditioning" may dominate the clinical picture contributing substantially to debility and incapacitation. Multiple studies5,18 have documented low health related quality of life in patients with POTS, with scores comparable to those seen in patients with congestive heart failure. Many patients have bowel irregularities and have been co-diagnosed with irritable bowel syndrome, and some have abnormalities of sudomotor regulation.19 About 80% of female patients report an exacerbation of symptoms around menstruation.20
Patients with POTS can often seem anxious in clinic. However, a misinterpretation of physical symptoms such as tachycardia and tremulousness might account for some of this apparent anxiety. When formally assessed, POTS patients did not have a higher incidence of major depressive disorder, anxiety disorders, or substance abuse than the general population.6 Using the Anxiety Sensitivity Index, there was a trend toward less anxiety in POTS patients than the general population,6 and the elevations in POTS correspond to blood pooling in the lower extremities, and not to anticipatory anxiety.21
Investigation of POTS
The evaluation of a patient with POTS starts with a detailed history and physical examination looking for common features outlined above. A pheochromocytoma can mimic POTS (or vice versa) because of the paroxysms of hyperadrenergic symptoms including palpitation, although pheochromocytoma patients are more likely to have these symptoms while supine than POTS patients. Plasma or urinary metanephrines22 can screen for pheochromocytoma. A routine CBC and electrolyte panel can exclude severe anemia or gross electrolyte disturbances.
The tachycardia in POTS patients should originate from the sinus node, and should develop and resolve relatively gradually with changes in posture. An electrocardiogram should be routinely performed to exclude the presence of an accessory bypass tract or other abnormalities of cardiac conduction. If the patient describes a paroxysmal tachycardia with a sudden onset and offset, especially in the supine or seated positions, then a Holter monitor or event recorder may be needed to exclude a reentrant tachycardia. It is essential that monitoring be continued as long as possible to capture a clinically relevant event. Left ventricular function must be normal for a diagnosis of POTS. A cardiomyopathy (e.g., peripartum) could mimic a POTS presentation.
Other testing may be reserved for referral centers. With formal autonomic nervous system testing, POTS patients often have preserved vagal function and a vigorous pressor response to the Valsalva maneuver, with an exaggerated blood pressure fall, recovery and overshoot both before and after release.23 Upright plasma norepinephrine (after at least 5-10 minutes of standing or tilting) is frequently elevated (>600 pg/ml) in POTS patients, reflecting the exaggerated neural sympathetic tone that is often present in these patients. Formal cardiopulmonary exercise testing can be useful for objective documentation of exercise capacity, and to serially quantify functional capacity over time. Since the blood volume is low in many patients with POTS,24,25 formal assessment with nuclear medicine tests may help to focus the treatment plan.
Treatment of POTS
Treatment efforts should begin by correcting reversible causes and optimizing chronic disease management. Patient education is important. If there has been a bout of prolonged bed rest, symptoms should gradually improve as patients recondition themselves to upright posture. POTS patients should avoid aggravating factors such as dehydration, and extreme heat. In an effort to optimize hydration, we patients are asked to consume 8-10 cups of water daily and to increase their sodium intake to up to 8-10 g/day. This should ideally be accomplished by dietary modification. We recommend panty-hose (waist high) style compression stockings with 30-40 mmHg of counter-pressure to minimize peripheral venous pooling and to enhance venous return. Elevating the head of the bed up on blocks 4-6 inches may also be helpful to facilitate expansion of the plasma volume.26
Radiofrequency ablation may be needed to treat reentrant supraventricular tachyarrhythmia, but radiofrequency sinus node modification for the sinus tachycardia of POTS is not recommended as this often makes the patient's symptoms worse (and occasionally pacemaker dependent).
Exercise
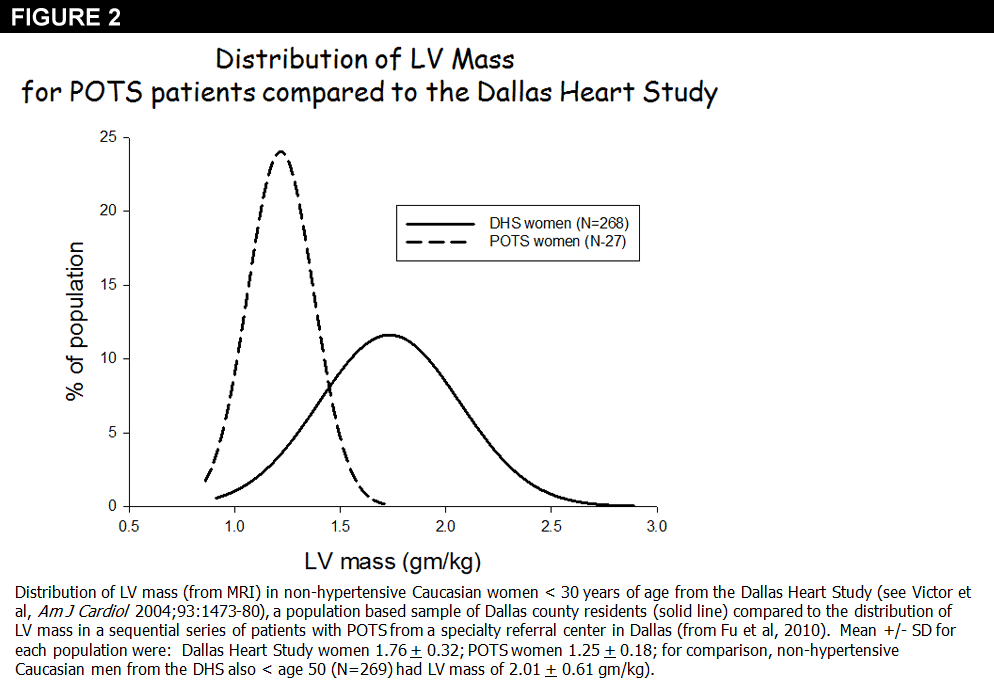
Patients with POTS have a small left ventricular mass (LV), LV end diastolic volume, and low upright stroke volume compared to normal controls when matched for gender (Figure 2);25,27 plasma volume and total blood volume are also low. Together, these cardiovascular characteristics are similar to what is seen after bedrest,13,14 and opposite to what is observed in athletes.28 Indeed, the high upright HR is proportional to this low upright SV suggesting that the orthostatic tachycardia is a normal autonomic response to the hemodynamics of the upright posture25 as is seen in astronauts.29 When this "cardiovascular deconditioning" from bed rest is prevented by supine or semi-recumbent exercise combined with volume repletion, the orthostatic intolerance is completely prevented.30,31 Therefore exercise training is a reasonable first line therapy for many patients with POTS.
Exercise has long been advised generically to POTS patients. Unfortunately, most POTS patients may not be able to tolerate upright exercise like a treadmill or elliptical machine, and report feeling debilitated for days post-exertion, limiting compliance with their exercise regimen. Anecdotally, patients who did exercise seemed to have a better long-term prognosis, but it was not certain if this was due to the exercise itself or due to a selection bias based on their ability to exercise.
Fu et al.25 recently administered a structured 3 month exercise program to 19 patients with POTS. This was a structured program that included primarily aerobic cardiovascular training, but also some resistance training involving primarily the leg muscles. The exercise program was detailed with individual training calendars developed for each patient, and when possible, it was done in a supervised setting. A key feature of this program was that patients were initially encouraged to perform all exercise in the seated position which dissociates the exercise induced tachycardia from the gravity induced tachycardia that is a problem in these patients. Recommended exercises included use of a rowing machine (which causes the most vigorous cardiac hypertrophy among all sports32 because of its unique combination of static and dynamic exercise;33 recumbent cycling or swimming are also effective.
Importantly, the Fu et al.25 exercise intervention reduced orthostatic tachycardia and improved quality of life, despite the relatively short duration. Physiological parameters such as blood volume, stroke volume and LV mass all improved over the 3 months, as did exercise tolerance, and the hemodynamic response to exercise.34 This study elegantly showed that exercise training is an important intervention in this population, and not just the ability to exercise. The Fu group is nearing completion of a much larger international registry of 250 patients, for whom the exercise intervention has been applied in the community instead of a carefully controlled research trial. Preliminary results have been presented recently and are very encouraging with a 73% "cure" rate meaning no longer meeting criteria for POTS after the intervention.35
Pharmacological Treatment of POTS
The initial pharmacological approach is to withdraw medications that might be predisposing to tachycardia (such as diuretics, vasodilators, and norepinephrine transporter blockers). Some oral contraceptives include drosperinone as the progestin, which is a spironolactone analogue. The use of pharmacological agents should not be viewed as a replacement for an exercise program, but as an adjunct to an exercise program.
Beta-adrenergic blockers are commonly used in cardiology clinics to control tachycardia, but tolerance can be a problem in many patients with POTS. While reducing the HR in POTS would be useful if the tachycardia was "over-compensation" for a physiological stimuli (i.e., a truly hyperdynamic circulation), but could be counter-productive if the HR increase in POTS were purely compensatory (e.g., low stroke volume). We have found low dose propranolol (10-20 mg PO TID-QID) to be very effective at lowering standing HR and improving symptoms acutely in POTS patients,36 while more complete beta-blockade was less well tolerated.36 Long-acting propranolol in the chronic setting was as effective as exercise at lowering standing HR, but did not improve quality of life in POTS patients.37 A non-selective beta blocker like propranolol may be more effective than a selective beta blocker like metoprolol since it also will block beta-2 adrenoreceptor mediated vasodilation.
In patients in whom the presence of hypovolemia is either known or strongly suspected, fludrocortisone (aldosterone analogue) is often used. Through enhanced sodium retention, it should expand the plasma volume, although clinical data are lacking. Adverse effects can include hypokalemia (which may be profound, especially when combined with Na+ loading), worsening headaches, acne, and fluid retention with edema.
Other medications used for POTS include midodrine, pyridostigmine and central sympatholytics. Midodrine is a peripheral alpha-1 adrenergic agonist that is a vasoconstrictor and venoconstrictor. Midodrine can cause scalp tingling, goose pimples, or headaches, which can limit its tolerability. Pyridostigmine is a peripheral acetylcholinesterase inhibitor that can increase the levels of synaptic acetylcholine at both the autonomic ganglia and the peripheral muscarinic parasympathetic receptors. Pyridostigmine significantly restrains HR in response to standing in POTS patients,38 and 30-60 mg PO TID resulted in chronic symptom improvement in ~50% of POTS patients.39 Pyridostigmine can enhance bowel motility, and this can lead to discontinuation of the drug in ~20%.39 Central sympatholytic agents can be useful in patients with are very hyperadrenergic with their POTS. Clonidine is an alpha-2 adrenergic agonist that acts centrally to decrease sympathetic nervous system outflow. Clonidine 0.1-0.2 mg PO BID-TID (eventually switched to a long-acting patch) can stabilize HR and BP, although α-methyldopa 125-250 mg PO BID (a false neurotransmitter) may be better tolerated due to its longer half-life. Unfortunately, both drugs can cause drowsiness, fatigue and worsen the mental clouding of some patients.40
Conclusions
POTS can produce substantial disability among previously healthy people. Patients with POTS demonstrate a HR increase of ≥30 bpm (≥40 bpm in children) within 10 min of standing, are often hyperadrenergic, and are quite symptomatic. Many patients suffer from a low stroke volume in the upright position, and in the chronic state, the disability may be dominated by a deconditioning phenotype. The focus of therapy should be an exercise reconditioning program, including both aerobic and resistance training, with an emphasis on non-upright exercises such as rowing machines, recumbent cycles and swimming. Pharmacological therapies targeting hypovolemia and the excess sympathetic nervous system activation may help relieve symptoms.
References
- Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology 1993;43:132-137.
- Raj SR. The Postural Tachycardia Syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J 2006;6:84-99.
- Singer W, Sletten DM, Opfer-Gehrking TL, Brands CK, Fischer PR, Low PA. Postural Tachycardia in Children and Adolescents: What is Abnormal? J Pediatr 2012;160:746-752.
- Brewster JA, Garland EM, Biaggioni I et al. Diurnal variability in orthostatic tachycardia: implications for the postural tachycardia syndrome. Clin Sci (Lond) 2012;122:25-31.
- Bagai K, Song Y, Ling JF et al. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med 2011;7:204-210.
- Raj V, Haman KL, Raj SR et al. Psychiatric profile and attention deficits in postural tachycardia syndrome. J Neurol Neurosurg Psychiatry 2009;80:339-344.
- Friesinger GC, Biern RO, Likar I, Mason RE. Exercise electrocardiography and vasoregulatory abnormalities. Am J Cardiol 1972;30:733-740.
- Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology 2007;69:790-798.
- Thieben MJ, Sandroni P, Sletten DM et al. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc 2007;82:308-313.
- Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol 2005;289:R109-R116.
- Fu Q, Okazaki K, Shibata S et al. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol 2009;587:2019-2031.
- Fu Q, Vangundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Menstrual cycle affects renal-adrenal and hemodynamic responses during prolonged standing in the postural orthostatic tachycardia syndrome. Hypertension 2010;56:82-90.
- Perhonen MA, Franco F, Lane LD et al. Cardiac atrophy after bed rest and spaceflight. J Appl Physiol 2001;91:645-653.
- Dorfman TA, Levine BD, Tillery T et al. Cardiac atrophy in women following bed rest. J Appl Physiol 2007;103:8-16.
- Perhonen MA, Zuckerman JH, Levine BD. Deterioration of left ventricular chamber performance after bed rest : "cardiovascular deconditioning" or hypovolemia? Circulation 2001;103:1851-1857.
- Buckey JC, Jr., Lane LD, Levine BD et al. Orthostatic intolerance after spaceflight. J Appl Physiol 1996;81:7-18.
- Gaffney FA, Nixon JV, Karlsson ES, Campbell W, Dowdey AB, Blomqvist CG. Cardiovascular deconditioning produced by 20 hours of bedrest with head-down tilt (-5 degrees) in middle-aged healthy men. Am J Cardiol 1985;56:634-638.
- Benrud-Larson LM, Dewar MS, Sandroni P, Rummans TA, Haythornthwaite JA, Low PA. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc 2002;77:531-537.
- Stewart JM, Medow MS, Glover JL, Montgomery LD. Persistent Splanchnic Hyperemia during Upright tilt in Postural Tachycardia Syndrome. Am J Physiol Heart Circ Physiol 2005.
- Peggs KJ, Nguyen H, Enayat D, Keller NR, Al-Hendy A, Raj SR. Gynecologic disorders and menstrual cycle lightheadedness in postural tachycardia syndrome. Int J Gynaecol Obstet 2012;118:242-246.
- Masuki S, Eisenach JH, Johnson CP et al. Excessive heart rate response to orthostatic stress in postural tachycardia syndrome is not caused by anxiety. J Appl Physiol 2007;102:896-903.
- Manger WM, Eisenhofer G. Pheochromocytoma: diagnosis and management update. Curr Hypertens Rep 2004;6:477-484.
- Shibao C, Arzubiaga C, Roberts LJ et al. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension 2005;45:385-390.
- Raj SR, Robertson D. Blood volume perturbations in the postural tachycardia syndrome. Am J Med Sci 2007;334:57-60.
- Fu Q, Vangundy TB, Galbreath MM et al. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol 2010;55:2858-2868.
- Wieling W, Colman N, Krediet CT, Freeman R. Nonpharmacological treatment of reflex syncope. Clin Auton Res 2004;14 Suppl 1:62-70.
- Victor RG, Haley RW, Willett DL et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 2004;93:1473-1480.
- Weiner RB, Baggish AL. Exercise-induced cardiac remodeling. Prog Cardiovasc Dis 2012;54:380-386.
- Levine BD, Pawelczyk JA, Ertl AC et al. Human muscle sympathetic neural and haemodynamic responses to tilt following spaceflight. J Physiol 2002;538:331-340.
- Shibata S, Perhonen M, Levine BD. Supine cycling plus volume loading prevent cardiovascular deconditioning during bed rest. J Appl Physiol 2010;108:1177-1186.
- Hastings JL, Krainski F, Snell PG et al. Effect of rowing ergometry and oral volume loading on cardiovascular structure and function during bed rest. J Appl Physiol 2012;112:1735-1743.
- Pelliccia A, Maron BJ, Spataro A, Proschan MA, Spirito P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Med 1991;324:295-301.
- Clifford PS, Hanel B, Secher NH. Arterial blood pressure response to rowing. Med Sci Sports Exerc 1994;26:715-719.
- Shibata S, Fu Q, Bivens TB, Hastings JL, Wang W, Levine BD. Short-term exercise training improves the cardiovascular response to exercise in the postural orthostatic tachycardia syndrome. J Physiol 2012;590:3495-3505.
- George SA, Bivens TB, Hendrickson D, Galbreath MM, Fu Q, Levine BD. Gravitational Based Therapy for POTS: An International Registry Evaluating the Success of a Structured, Graduated Exercise Program Administered in a Community Setting [abstract]George SA, Bivens TB, Hendrickson D, Galbreath MM, Fu Q, Levine BD. Circulation 2012;126:A16542
- Raj SR, Black BK, Biaggioni I et al. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation 2009;120:725-734.
- Fu Q, Vangundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Exercise training versus propranolol in the treatment of the postural orthostatic tachycardia syndrome. Hypertension 2011;58:167-175.
- Raj SR, Black BK, Biaggioni I, Harris PA, Robertson D. Acetylcholinesterase inhibition improves tachycardia in postural tachycardia syndrome. Circulation 2005;111:2734-2740.
- Kanjwal K, Karabin B, Sheikh M et al. Pyridostigmine in the treatment of postural orthostatic tachycardia: a single-center experience. Pacing Clin Electrophysiol 2011;34:750-755.
- Jacob G, Biaggioni I. Idiopathic orthostatic intolerance and postural tachycardia syndromes. Am J Med Sci 1999;317:88-101.
< Back to Listings
