Cardiotoxicity: An Unexpected Consequence of HER2-Targeted Therapies
Introduction
Human epidermal growth factor receptor- (HER2-) targeted therapies trastuzumab, lapatinib, pertuzumab, and ado-trastuzumab emtansine have revolutionized the management of breast cancer. The HER2 gene is overexpressed in 25-30% of breast cancers, leading to abnormally high levels of HER2 protein in malignant cells.1,2 HER2-positive breast cancer is aggressive and associated with decreased time to recurrence and survival.1
Trastuzumab is a humanized monoclonal antibody targeted against HER2 protein. In 2001, the first phase III clinical trial of trastuzumab in addition to standard chemotherapy for metastatic breast cancer showed longer time to disease progression, higher rate of objective response, lower rate of death at 1 year, longer survival, and 20% reduction in risk of death.3 Trastuzumab in conjunction with adjuvant chemotherapy in patients with surgically removed HER2-positive breast cancer was associated with decreased rates of recurrence, decrease in distant recurrence, and a 33% reduction in risk of death.4 The principle adverse event noted in these trials was cardiac dysfunction with an incidence of 27% in metastatic patients who received anthracycline, cyclophosphamide and trastuzumab and an incidence of New York Heart Association Class III or IV heart failure (HF) of 4.1% in the adjuvant setting.3,4 Cardiac dysfunction with trastuzumab is often asymptomatic but can be symptomatic.5,6 Trastuzumab is currently approved for use in adjuvant breast cancer, metastatic breast cancer, and metastatic gastric cancer.6 Per the package insert, trastuzumab can cause "left ventricular dysfunction, arrhythmias, hypertension, disabling cardiac failure, cardiomyopathy and cardiac death." Cardiac dysfunction, the most commonly seen side effect in clinical practice, is the focus of this brief review.7
Proposed Mechanism, Reversibility, and Risk Factors
Although the exact mechanism behind trastuzumab-induced cardiotoxicity has yet to be elucidated, it is thought that the neuregulin (NRG) ERBB pathway plays a key role. HER2 is one of the tyrosine kinases of the epidermal growth factor receptor or ERBB family. NRG is a ligand to the ERBB receptors, and NRG-ERBB signaling is involved in cardiac development and physiology.8 Spurred by development of cardiotoxicity with trastuzumab, the NRG-ERBB axis was found to be connected to the stress response of the heart.9 Patients with stable chronic HF have high levels of circulating NRG.10 Reduced ERBB signaling in the failing heart has led to work looking at the use of recombinant human NRG in patients with stable chronic HF.11 Although inhibition of the NRG-ERBB pathway in cardiomyocytes with trastuzumab leading to cardiac dysfunction seems plausible, further work is needed to prove this hypothesis.12
Cardiotoxicity from trastuzumab is largely reversible and therefore characterized as causing type II chemotherapy-related cardiac dysfunction.13 Doxorubicin is the prototypical example of a type I chemotherapy-related cardiac dysfunction agent and results in irreversible, dose-dependent myocardial damage. In contrast, trastuzumab appears to cause myocardial dysfunction (not damage), has a high likelihood of being reversible, is not dose dependent, and carries a low likelihood of late sequential stress-related cardiac dysfunction. In addition, rechallenge can be possible depending on the clinical scenario.13
The greatest risk of cardiotoxicity from trastuzumab is in patients receiving concurrent anthracycline.6,14 Other risk factors for trastuzumab-induced cardiotoxicity include older age, diabetes mellitus, decreased glomerular filtration rate, use of antihypertensives, and history of heart disease. Studies have suggested that impaired left ventricular (LV) dysfunction and low baseline LV ejection fraction (LVEF) are also risk factors for cardiotoxicity.15
Incidence of Cardiotoxicity With Lapatinib, Pertuzumab, and Ado-Trastuzumab Screening and Management
Because not all HER2-positive breast cancer will respond to trastuzumab and because metastatic disease often eventually progresses, additional HER2-targeted therapies have been developed. Incidence of cardiotoxicity for all HER2-targeted therapies can be variable depending on the associated treatment regimen and definition of cardiotoxicity used. Therefore the rates in large trials are presented below to provide a sense of the scope of the issue.
Lapatinib is an oral reversible inhibitor of epidermal growth factor receptor HER1 and HER2 tyrosine kinases.16 In pooled analysis of 3,689 patients receiving lapatinib enrolled in 44 clinical trials, the incidence of a cardiac event was 1.6%. Study-defined cardiac events were asymptomatic (LVEF decrease ≥ 20% from baseline or below the institution's lower limit of normal without symptoms) or symptomatic (symptomatic congestive HF responsive to intervention with ejection fraction of 20-39% (LV systolic dysfunction [National Cancer Institute grade 3] or refractory/poorly controlled congestive HF with ejection fraction < 20% necessitating consideration for ventricular assist device, surgery, or heart transplant [National Cancer Institute grade 4]).17 Eighty-three percent of patients were asymptomatic.18
Pertuzumab is a recombinant humanized monoclonal antibody that binds to a different domain of HER2 than trastuzumab and works synergistically with trastuzumab.19,20 CLEOPATRA (A Study to Evaluate Pertuzumab + Trastuzumab + Docetaxel vs. Placebo + Trastuzumab + Docetaxel in Previously Untreated HER2-positive Metastatic Breast Cancer) was a phase III randomized, double-blind placebo controlled trial investigating pertuzumab + trastuzumab + docetaxel to treat metastatic breast cancer that showed significantly improved survival with pertuzumab.21 Long-term follow-up with a median of 50 months in CLEOPATRA revealed a 6.6% incidence of LV dysfunction in the pertuzumab group versus 8.6% in the control group and 1 episode of symptomatic LV dysfunction in the pertuzumab group, which resolved after 3 months with discontinuation of pertuzumab and trastuzumab. Compared with the control group, fewer patients in the pertuzumab group (7.4 vs. 6.1%) had a reduction in LVEF ≥ 10% from baseline to less than 50%. The rate of reversal was higher in the pertuzumab group (87.5 vs. 78.6% in controls).22
Ado-trastuzumab emtansine is an antibody-drug conjugate that targets HER2-positive tumors.23 In EMILIA (An Open-label Study of Trastuzumab Emtansine [T-DM1] vs Capecitabine + Lapatinib in Patients With HER2-positive Locally Advanced or Metastatic Breast Cancer), a phase III randomized, open-label trial comparing ado-trastuzumab with lapatinib + capecitabine to treat metastatic breast cancer, 97.1% of patients in the ado-trastuzumab arm and 93.0% of patients in the lapatinib + capecitabine arm maintained an LVEF ≥ 45% through treatment. Of the 481 patients in the ado-trastuzumab group, 1.7% developed an LVEF < 50% and at least 15% below baseline versus 1.6% in the lapatinib + capecitabine group. One patient in the ado-trastuzumab arm developed grade 3 LV systolic dysfunction.24 Additional HER2-targeted therapies, neratinib and afatinib, are available; however, their role in the treatment of breast cancer is still under investigation.16 Afatinib is approved for first-line treatment of metastatic nonsmall-cell lung cancer, and treatment should be stopped if patients develop symptomatic LV dysfunction; however, there are no recommended LVEF monitoring guidelines in the package insert.25
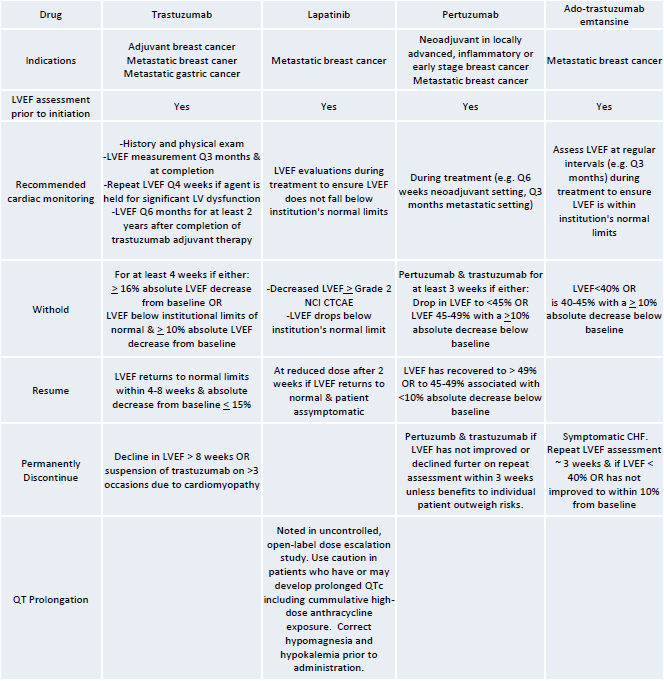
Given the risk of cardiac dysfunction, the Food and Drug Administration (FDA) recommends screening by echocardiogram or multigated acquisition at baseline and at intervals during treatment, which is outlined in Table 1. QTc should be monitored in patients on lapatinib.
Table 1
Biomarkers, particularly troponin I, and echo parameters such as strain imaging have been studied in an effort to predict trastuzumab cardiotoxicity.29,30 Consensus guidelines from the American Society of Echocardiography and the European Association of Cardiovascular Imaging for patients with cancer state that global strain is the optimal parameter for early detection of subclinical LV dysfunction.30 Randomized trials to assess the effectiveness of angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers, and beta blockers to prevent trastuzumab-induced cardiotoxicity are underway.31,32 Management of LV dysfunction resulting from HER2-targeted therapy has not been studied in large prospective clinical trials.33 ACEIs and beta blockers have been used in case series and small trials of patients with trastuzumab-induced LV dysfunction with promising results.34,35 It is not clear how long after treatment patients should remain on these drugs because this has also not been directly studied.7 In keeping with the 2013 American College of Cardiology Foundation and American Heart Association HF guidelines, patients who have received HER2-targeted therapies should be counseled that they have stage A HF.36
Conclusion
In summary, HER2-targeted therapies have transformed treatment options in breast cancer. HER2 is expressed in tumor cells and also in cardiomyocytes, leading to cardiac dysfunction when these cancer therapies are used. Cardiac dysfunction is the primary clinical cardiac side effect seen with HER2-targeted therapies, and incidence differs by agent. The FDA recommends baseline and interval screening for LV dysfunction in patients receiving HER2-targeted therapy. ACEIs and beta blockers are the mainstays of therapy for LV dysfunction resulting from HER2-targeted therapies, although data from large trials are lacking. Finally, counseling patients on their exposure to cardiotoxic chemotherapy is an important piece of survivorship care.
References
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire, WL. Human breast cancer: correlation of relapse and survival with amplification of HER-2/neu oncogene. Science 1987;235:177-182.
- Bilous M, Ades C, Armes J, et al. Predicting the HER2 status of breast cancer from basic histopathology data: an analysis of 1500 breast cancers as part of the HER2000 International Study. Breast 2003;12:92-8.
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92.
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673-84.
- Tarantini L, Cioffi G, Gori S, et al. Trastuzumab adjuvant chemotherapy and cardiotoxicity in real-life women with breast cancer. J Card Fail 2012;18:113-9.
- Food and Drug Administration prescribing information on HERCEPTIN (trastuzumab), revised 2010, http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf (accessed January 9, 2016).
- Suter TM, Ewer MS. Cancer drugs and the heart: importance and management. Eur Heart J 2013;34:1102-11.
- Lemmens K, Doggen K, De Keulenaer GW. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation 2007;116:954-60.
- Cote GM, Sawyer DB, Chabner BA. ERBB2 inhibition and heart failure. N Engl J Med 2012;367:2150-3.
- Ky B, Kimmel SE, Safa RN, et al. Neuregulin-1 beta is associated with disease severity and adverse outcomes in chronic heart failure. Circulation 2009;120:310-7.
- Jabbour A, Hayward CS, Keogh AM, et al. Parental administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail 2011;13:83-92.
- Ky B, Vejpongsa P, Yeh ET, Force T, Moslehi JJ. Emerging paradigms in cardiomyopathies associated with cancer therapies. Circ Res 2013;113:754-64.
- Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol 2005;23:2900-2.
- Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 2002;20:1215-21.
- Onitilo AA, Engel JM, Stankowski RV. Cardiovascular toxicity associated with adjuvant trastuzumab therapy: prevalence, patient characteristics, and risk factors. Ther Adv Drug Saf 2014;5:154-66.
- Sendur MA, Aksoy S, Altundag K. Cardiotoxicity of novel HER2-targeted therapies. Curr Med Res Opin 2013;29:1015-24.
- National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE), http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf (accessed January 11, 2016).
- Perez EA, Koehler M, Byrne J, Preston AJ, Rappold E, Ewer MS. Cardiac safety of lapatinib: pooled analysis of 3689 patients enrolled in clinical trials. Mayo Clin Proc 2008;83:679-86.
- Adams CW, Allison DE, Flagella K, et al. Humanization of recombinant monoclonal antibody to produce a therapeutic HER dimerization inhibitor, pertuzumab. Cancer Immunol Immunother 2006;55:717-27.
- Cortes J, Fumoleau P, Bianchi GV, et al. Pertuzumab monotherapy after trastuzumab-based treatment and subsequent reintroduction of trastuzumab: activity and tolerability in patients with advanced human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2012;30:1594-600.
- Swain SM, Kim SB, Cortes J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomized, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2013;14:461-71.
- Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015;372:724-34.
- Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 2008;68:9280-90.
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783-91.
- Food and Drug Administration prescribing information on GILOTRIF(afatinib), 2013, https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/201292s000lbl.pdf (accessed January 11, 2016).
- . Food and Drug Administration prescribing information on TYKERB (lapatinib), 2007, http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022059s007lbl.pdf (accessed January 9, 2016).
- Food and Drug Administration prescribing information on PERJETA (pertuzumab), revised 2013, http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125409s051lbl.pdf (accessed January 9, 2016).
- Food and Drug Administration prescribing information on KADCYLA (ado-trastuzumab emtansine), http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125427lbl.pdf (accessed January 9, 2016).
- Stevens PL, Lenihan DJ. Cardiotoxicity due to chemotherapy: the role of biomarkers. Curr Cardiol Rep 2015;17:603.
- Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014;27:911-39.
- Pituskin E, Haykowsky M, Mackey JR, et al. Rationale and design of the Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research Trial (MANTICORE101—Breast): a randomized, placebo-controlled trial to determine if conventional heart failure pharmacotherapy can prevent trastuzumab-mediated left ventricular remodeling among patients with HER2+ early breast cancer using cardiac MRI. BMC Cancer 2011;11:318.
- Heck SL, Gulati G, Ree AH, et al. Rational and design of the prevention of cardiac dysfunction during an adjuvant breast cancer therapy (PRADA) trial. Cardiology 2012;123:240-7.
- Zagar TM, Cardinale DM, Marks LB. Breast cancer therapy-associated cardiovascular disease. Nat Rev Clin Oncol 2015;13:172-84.
- Ewer MS, Vooletich MT, Durund JB, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol 2005;23:7820-6.
- Oliva S, Cioffi G, Frattini S, et al. Administration of angiotensin-converting enzyme inhibitors and β-blockers during adjuvant trastuzumab chemotherapy for nonmetastatic breast cancer: marker of risk or cardioprotection in the real world? Oncologist 2012;17:917-24.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147-239.
Keywords: Angiotensin Receptor Antagonists, Angiotensin-Converting Enzyme Inhibitors, Antibodies, Monoclonal, Humanized, Antihypertensive Agents, Arrhythmias, Cardiac, Biomarkers, Breast Neoplasms, Cardiomyopathies, Cardiotoxicity, Chemotherapy, Adjuvant, Cyclophosphamide, Diabetes Mellitus, Disease Progression, Doxorubicin, Echocardiography, Genes, erbB-2, Glomerular Filtration Rate, Heart Diseases, Heart Failure, Heart Transplantation, Heart Transplantation, Heart-Assist Devices, Hypertension, Lung Neoplasms, Maytansine, Myocytes, Cardiac, Neuregulins, Pharmaceutical Preparations, Prospective Studies, Protein-Tyrosine Kinases, Quinazolines, Receptor, Epidermal Growth Factor, Risk Factors, Stomach Neoplasms, Survival Rate, Taxoids, Troponin I, Ventricular Dysfunction, Left
< Back to Listings