For the FITs | Spontaneous Coronary Artery Dissection: A Primer
Spontaneous coronary artery dissection (SCAD) is an important cause of myocardial ischemia, especially in the young, because of intramural hematoma (IMH) or intimal dissection. It is not associated with atherosclerosis or trauma.
Since its first description by Pretty in 1931,1 an increasing number of cases has been described in the past decade due to registries as well as the use of coronary angiograms and intracoronary imaging. There are no randomized, prospective trials to guide management and much of the available data are based on observational and retrospective analyses.
As a result, there continues to be opportunities to improve recognition and management of this potentially devastating condition.
Clinical Case
A 29-year-old woman who was 10 days postpartum presented with chest pain, 1 mm ST-segment depressions in the anterolateral leads and troponin-I of 11.4 ng/mL (normal range <0.01 ng/mL). Coronary angiography revealed a 90 percent occlusion of a long lesion in the mid ramus that was diagnosed as SCAD. Given that the patient was hemodynamically stable, medical therapy with aspirin, clopidogrel and metoprolol was recommended. Counseling was provided on risk of recurrence with future pregnancies and screening for fibromuscular dysplasia (FMD). She is doing well at her one-year follow-up.
Incidence
Acute coronary syndrome (ACS) is the most common presentation, predominantly in women between 45-53 years of age, but it can be seen in the extremes of age. Overall, it occurs in about 1-4 percent of all cases of ACS, but the incidence in women <50 years of age presenting with myocardial infarction (MI) is around 35 percent.2,3 It is the most common cause of pregnancy-associated MI.
Pathogenesis
Two theories exist: a primary event with the development of an intimal tear, creating a false lumen (inside-out) or a spontaneous hemorrhage of the vasa vasorum within the vessel wall, forming an IMH, causing a secondary intimal tear (outside-in).4 Isolated IMH has a higher risk for progression and clinical deterioration.
Coronary Distribution
SCAD usually involves a single vessel, in the mid-to-distal segment. The left anterior descending artery is the most commonly involved (32-46 percent),5 followed by the left circumflex and right coronary artery. In 10 percent of cases, proximal and multivessel involvement have been seen.
Types
Based on angiographic appearance:
- Type 1: "pathognomonic" appearance of multiple radiolucent and extraluminal contrast staining; comprises a quarter of cases
- Type 2: long (20-30 mm) diffuse smooth narrowing; most common type with two-third of cases
- Type 3: focal or tubular stenosis (usually <20 mm) that mimics atherosclerosis; about 5 percent of cases6
Etiology
The etiology for SCAD remains unknown. It is associated with conditions that weaken vessel walls such as FMD, connective tissue disorders (e.g., Marfan's, Ehlers Danlos, Loeys-Dietz), systemic inflammatory conditions (e.g., lupus, rheumatoid arthritis, inflammatory bowel disease) and pregnancy.
FMD is a nonatherosclerotic, noninflammatory vascular disease affecting medium-sized arteries and manifests as arterial stenosis, aneurysms or dissection. The first association between SCAD and FMD was described in 2005 and can range from 25-86 percent2 of SCAD cases.
Precipitants
The most commonly reported precipitants are emotional stress in women and physical stress in men. It is possible that the catecholamine surge during these events causes arterial shear stress, which in addition to hypertension and tachycardia contributes to spontaneous hematoma and dissection. Hormonal therapy, stressors like intense retching, vomiting, straining with bowel movements, recreational drugs and pregnancy were also documented.
Diagnosis
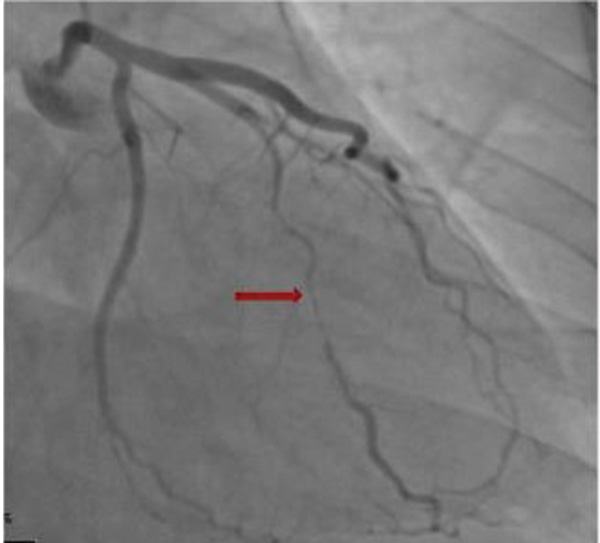 Figure. A 29-year-old postpartum female patient presenting with chest pain and SCAD of ramus intermedius. Note the long, narrowed segment involving the mid segment, typical for type 2 SCAD.
Figure. A 29-year-old postpartum female patient presenting with chest pain and SCAD of ramus intermedius. Note the long, narrowed segment involving the mid segment, typical for type 2 SCAD.
Given the young age of presentation and absence of atherosclerotic risk factors, the diagnosis may be missed, with patients being discharged from the emergency room without further work-up.
Coronary angiography is the first-line test because of its ability to provide a definitive diagnosis. There is however an associated risk of iatrogenic catheter-induced coronary artery dissection due to the friable artery (incidence of 3.4 percent, vs. <0.2 percent in the general population2).
Intracoronary imaging (IVUS or optical coherence tomography7) may be required to differentiate from atherosclerosis, especially in type 2 or 3 SCAD, but its use is limited due to cost, limited availability and the risk of worsening the dissection with the wire of the imaging catheter or hydraulic extension with contrast injection for OCT.
With coronary computed tomography angiography (CCTA), SCAD vessels can appear as having dual lumens as opposed to calcifications and plaque seen in atherosclerosis. CCTA has low spatial and temporal resolution, and lack of staining in the absence of intimal disruption can result in false negatives, limiting its utility in low to moderate risk patients.
Management
An ideal management strategy has yet to be determined. In one series, 80 percent of dissections healed with conservative therapy alone.8 Early complications can occur in 5-10 percent of patients, usually within the first five days due to progression of IMH and dissection. Inpatient monitoring is recommended for at least 48 hours and ideally up to five days.
Conservative Management
- Long-term, low-dose aspirin.
- Role of clopidogrel, though debated, remains uncertain. May institute for 1-12 months after discussing its benefits and risks of bleeding.
- No role for a statin, unless patient has dyslipidemia, due to similar incidence of recurrence with and without statin therapy.
- Hypertension is associated with twofold increased risk for recurrent SCAD.
- Beta-blockers reduce risk for recurrence and must be weighed against side effect profile.
- Avoid heparin, given IMH and risk for bleeding and propagation.
- No role for GPIIb/IIIa inhibitors.
- Angiotensin-converting enzyme inhibitors may be started if there is left ventricular dysfunction.2,8
Invasive Therapy
- Consider PCI or CABG in high-risk patients with ongoing ischemia, left main involvement and/or hemodynamic instability.
- PCI is associated with a high risk of complications and suboptimal success. Extension of dissection can result in spiral dissection of distal or proximal segments, including the left main. Given the lesions tend to be long, requiring longer stents, there is a higher risk for subsequent in-stent restenosis and stent thrombosis. IMH naturally resorbs over time and can result in subacute or late-stent malposition, increasing risk for stent thrombosis.
- If intervention is undertaken, consider the following:
- Avoid deep catheter engagement to reduce the risk for catheter-induced dissection.
- Radial approach is associated with a threefold higher risk for catheter-induced iatrogenic dissection vs. femoral access.
- Avoid non-coaxial views.
- Limit contrast use and the force of injection.
- Use long stents, covering 5-10 mm on both proximal and distal edges of IMH to accommodate propagation of IMH when compressed by the stent.
- Direct stenting without predilatation to reduce risk of IMH extension or balloon angioplasty alone.
- Cutting balloon fenestration of the IMH to allow decompression of false lumen blood pool into the true lumen.
- Multistent approach by sealing the dissections proximally and distally first, and then placing a stent in the mid segment.2
- Emergency CABG is required in left main involvement or failure of primary PCI. CABG has a high rate of both venous and arterial conduit failure due to subsequent healing of native vessels and competitive flow. CABG does not protect against recurrent SCAD.
Follow-Up
There is no role for routine follow-up with early angiography due to the risk of catheter-related trauma. Recurrent MI from recurrent SCAD has been seen in 30 percent at four to 10 year follow-up. There is a potential risk for recurrent SCAD in subsequent pregnancies, hence counseling must be undertaken. Given the association with FMD, a one-time CT from the head to pelvis, focusing on the carotids and renal arteries, may be considered.
Genetics
Fewer than 5 percent of cases have an association with inherited arteriopathies and connective tissue diseases. These patients should undergo further genetic testing as deemed appropriate. A genetic risk loci for SCAD has not been found and studies are currently in progress to identify any such associated mutations.
Cardiac Rehabilitation
Cardiac rehabilitation should be recommended regardless of patients' young age or lack of atherosclerosis. It has been shown to improve outcomes as well as provide social support, and alleviate emotional burden.9 The Vancouver General Hospital SCAD Program notes that exercise that maintains blood pressure under <130/80 mm Hg, heart rate of 50-70 percent of reserve and weight lifting up to 20 pounds for women and 50 pounds for men is considered safe. Patients should be advised to perform low resistance exercises and to avoid high-intensity, highly competitive contact sports.
Conclusion
SCAD continues to be underdiagnosed given the frequently young population and misdiagnosis as atherosclerosis. Conservative therapy is recommended, unless there is hemodynamic instability or ongoing ischemia. If considering PCI, keep in mind the high risk for iatrogenic dissection, in-stent restenosis and thrombosis. Patients should undergo risk factor modification, aggressive blood pressure management, counseling on future pregnancies to reduce recurrence and supervised cardiac rehabilitation.

This article was authored by Archana Kodali, MD, a cardiology fellow at Kettering Medical Center in Kettering, OH, where she'll continue her training next year as an interventional fellow.
References
- Pretty HC. Br Med J 1931;1:667.
- Hayes SN, Kim ESH, Saw J, et al. Circulation 2018;137:e523-e557.
- Hayes SN. Tex Heart Inst J 2014;41:295-8.
- Waterbury TM, Tweet MS, Hayes SN, et al. Circ Cardiovasc Interv 2018;11:e006772.
- Saw J, Humphries K, Aymong E, et al. J Am Coll Cardiol 2017;70:1148-58.
- Saw J. Catheter Cardiovasc Interv 2014;84:1115-22.
- Lettieri C, Zavalloni D, Rossini R, et al. Am J Cardiol 2015;116:66-73.
- Alfonso F, Paulo M, Lennie V, et al. JACC Cardiovasc Interv 2012;5:1062-70.
- Chou AY, Prakash R, Rajala J, et al. Can J Cardiol 2016;32:554-60.
Clinical Topics: Acute Coronary Syndromes, Anticoagulation Management, Arrhythmias and Clinical EP, Cardiac Surgery, Cardiovascular Care Team, Congenital Heart Disease and Pediatric Cardiology, Diabetes and Cardiometabolic Disease, Dyslipidemia, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Prevention, Vascular Medicine, Anticoagulation Management and ACS, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Aortic Surgery, Cardiac Surgery and Arrhythmias, Cardiac Surgery and CHD and Pediatrics, Cardiac Surgery and Heart Failure, Congenital Heart Disease, CHD and Pediatrics and Arrhythmias, CHD and Pediatrics and Imaging, CHD and Pediatrics and Interventions, CHD and Pediatrics and Prevention, CHD and Pediatrics and Quality Improvement, Nonstatins, Novel Agents, Statins, Interventions and ACS, Interventions and Imaging, Interventions and Structural Heart Disease, Interventions and Vascular Medicine, Angiography, Computed Tomography, Nuclear Imaging, Exercise, Hypertension, Stress
Keywords: ACC Publications, Cardiology Interventions, Acute Coronary Syndrome, Adrenergic beta-Antagonists, Aneurysm, Angiography, Angioplasty, Balloon, Coronary, Angiotensin-Converting Enzyme Inhibitors, Arthritis, Rheumatoid, Arthritis, Rheumatoid, Aspirin, Atherosclerosis, Blood Pressure, Cardiac Rehabilitation, Catecholamines, Chest Pain, Connective Tissue, Connective Tissue Diseases, Constriction, Pathologic, Coronary Angiography, Coronary Vessel Anomalies, Diabetes Mellitus, Type 2, Diagnostic Errors, Dyslipidemias, Emergency Service, Hospital, Exercise Therapy, Fibromuscular Dysplasia, Follow-Up Studies, Genetic Testing, Heart Rate, Hematoma, Heparin, Hospitals, General, Hydroxymethylglutaryl-CoA Reductase Inhibitors, Hypertension, Iatrogenic Disease, Inflammatory Bowel Diseases, Metoprolol, Myocardial Infarction, Postpartum Period, Pregnancy, Prospective Studies, Registries, Renal Artery, Retrospective Studies, Social Support, Risk Factors, Staining and Labeling, Stents, Illicit Drugs, Stress, Psychological, Tachycardia, Thrombosis, Ticlopidine, Tomography, X-Ray Computed, Troponin I, Vasa Vasorum, Ventricular Dysfunction, Left, Vomiting
< Back to Listings

