Lipoprotein(a) in Clinical Practice
Editor's Note: Commentary based on Wilson DP, Jacobson TA, Jones PH, et al. Use of Lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol 2019;13:374-92.
Introduction
The traditional lipid profile has served as a mainstay of atherosclerotic cardiovascular disease (ASCVD) risk assessment for decades. This tool became even more important as targeted therapies, such as statins and more recently ezetimibe and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, became available. Even with these advances in lipid management, and more broadly in targeting traditional cardiovascular risk factors, ongoing risk in many patients points to a need to investigate other contributors as possible therapeutic targets. Lipoprotein(a) (Lp[a]) is a promising biomarker to help refine current strategies of ASCVD risk assessment, and it is estimated to be elevated in approximately 20% of the world's population. Research on Lp(a) suggests it has added value in preventive medicine, and it is time for cardiologists and cardiovascular team members to consider using it routinely in their practice. Based on the mounting evidence, Wilson et al recently published a guideline on the clinical use of Lp(a) from the National Lipid Association, and herein we summarize key elements of their work.
What is Lipoprotein(a)?
Lipoprotein(a) is a low-density lipoprotein (LDL) particle with an added apolipoprotein(a) (apo[a]) attached to the apoplipoprotein(b) (apo[b]) component of the LDL particle via a disulfide bridge. The structure of Lp(a) is highly heterogeneous secondary to many different apo(a) isoforms within the population. Etiologically, it may have provided a survival advantage by aiding in wound healing and reducing bleeding, particularly in childbirth. An individual's Lp(a) level is 80-90% genetically determined in an autosomal codominant inheritance pattern with full expression by 1-2 years of age and adult-like levels achieved by approximately 5 years of age. Outside of acute inflammatory states, the Lp(a) level remains stable through an individual's lifetime regardless of lifestyle.
Early data suggest a link between Lp(a) level and both ASCVD and valvular aortic stenosis (VAS), but the exact pathophysiologic mechanism is not entirely clear. One current theory includes a two-pronged contribution by the Lp(a) molecule. Apo(a) has homology with plasminogen and has been shown in vitro to inhibit fibrinolysis. Therefore, it could hypothetically promote thrombosis at vulnerable arterial plaques or turbulent flow within stenosis causing obstruction or promoting emboli formation in VAS. The LDL-like portion may promote intimal cholesterol deposition but the cholesterol content in even very high levels of Lp(a) are below traditional LDL cutoffs and likely contribute less. Newer evidence suggests that oxidized phospholipids co-localize with Lp(a) molecules and together may promote endothelial dysfunction, inflammation and calcification in vasculature.
High quality evidence supports a link between Lp(a) levels and a variety of cardiovascular related outcomes. One meta-analysis showed an increased risk of CHD and MI with concentrations >30 mg/dL (62 nmol/L) while the INTERHEART trial showed Lp(a) >50 mg/dL was associated with increased risk of MI (OR 1.48; 95% CI 1.32-1.67; P < 0.001). The same meta-analysis showed increased risk of stroke at Lp(a) <50 mg/dL and another showed a 2x higher risk of ischemic stroke with smaller apo(a) isoforms [and higher Lp(a) concentrations]. Table 1 shows the risk of elevated Lp(a) on a variety of cardiovascular conditions based on large prospective, population-based studies and that these associations were also seen in Mendelian randomization studies. Finally, genome wide association studies focusing on genetic variation and risk of disease found that high Lp(a) concentrations confer the highest risk of ASCVD and VAS independent of other known causes and risk factors.
Table 1: Impact of elevated Lp(a) levels on various clinical outcomes based on large prospective, population-based studies and whether these conclusions were supported by large Mendelian randomization studies.
Measurement and Target Levels of Lp(a)
One of the main obstacles to the clinical use of Lp(a) is that its measurement and target levels have not been standardized. Several available assays report results in mass (mg/dL) instead of concentration (nmol/L), the latter of which is preferred. Unlike other lipids and lipoproteins, direct conversion between these two units is not possible because of the variable number of repeated units in different apo(a) isoforms, which leads to over- or underestimation depending on the particle size. Irrespective of the assay or units used, Lp(a) levels vary among ethnic groups and disease states, as well as whether measurements were from fresh or frozen samples, which have made published target levels inconsistent. Fortunately, all of these can be overcome by standardization of the assay and generated measurements. The guideline recommends reporting Lp(a) levels as concentration (nmol/L) using an assay calibrated against the WHO/International Federation of Clinical Chemistry and Laboratory Medicine secondary reference manual.
Based on the available studies, the guideline recommends a universal cut point of ≥100 nmol/L (approximately ≥50 mg/dL), which approximates the 80th percentile in the Caucasian U.S. populations. However, the use of this cut point remains a topic of debate amongst many experts in the lipid community and this likely stems from the lack of standardization and epidemiologic differences. This controversy is represented by the 2018 American Heart Association (ACC)/American Heart Association (AHA) Cholesterol Guidelines, which suggest high risk is ≥125 nmol/L (or ≥50 mg/dL). The cut off may change as additional studies are completed, recognizing that the cut off may vary based on risk, ethnicity, and comorbidities.
Which Patients Should Have Lp(a) Measured?
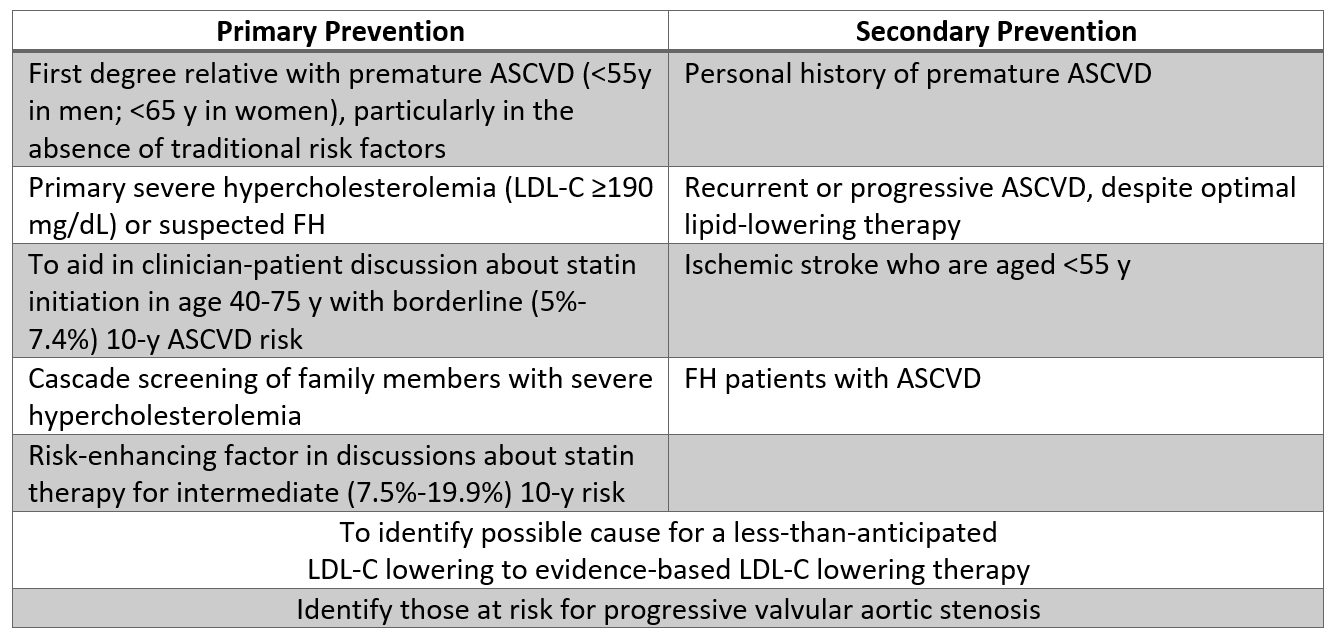
Assuming access to a WHO-standardized assay, select patients may benefit from Lp(a) testing after a shared decision-making discussion is completed. Based on this guideline, measuring Lp(a) may be reasonable in patients with a personal history of or first-degree relative with premature ASCVD (particularly if otherwise considered low-risk), and in severe hypercholesterolemia (LDL-C ≥190 mg/dL). Testing in these patients may trigger escalation of therapy, as described below. Additional indications where testing may be reasonable are listed in Table 2, most notably including patients where the LDL-C response to statins is less than anticipated, or in borderline-risk patients (5% to ≤ 7.5% 10-year ASCVD risk) who are particularly interested in reducing their ASCVD risk.
Table 2: Populations where Lp(a) testing may be reasonable based on current evidence.
Studies like a JUPITER (Justification for Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin) sub-analysis show Lp(a) is a strong predictor of risk when it remains elevated in patients who are already on a statin. However, no available data support that treating isolated, elevated Lp(a) leads to better clinical outcomes. If and when targeted Lp(a) treatment becomes available, it may be reasonable to start universal screening at a young age to determine if earlier preventive measures are indicated.
Interestingly, there may be a more acute effect as current evidence shows a link between elevated Lp(a) and ischemic stroke in youth. Therefore, it may be reasonable to measure Lp(a) in youth with genetically confirmed or clinically suspected familial hypercholesterolemia (FH), family history of first-degree relative with premature ASCVD, or ischemic stroke or a parent/sibling found to have elevated Lp(a). However, given that Lp(a) levels remain stable throughout one's life outside of acute inflammation, there is not much role serial measurement of Lp(a) levels at the present time.
How Do You Treat Elevated Lp(a)?
Despite the observed associations between elevated Lp(a) and ASCVD, there has yet to be a randomized controlled trial to see if targeted lowering of Lp(a) improves clinical outcomes. However, the Lp(a)-lowering effect of currently available therapies has been investigated.
Of the current available preventive therapies, only a limited number are effective in reducing Lp(a). In the pivotal trial of evolocumab (FOURIER – Further Cardiovascular Outcomes Researched With PCSK9 Inhibition in Subjects With Elevated Risk), a PCSK9 inhibitor, Lp(a) was reduced by 27% and the observed reduction in major adverse cardiovascular events (MACE) was greatest for those with the highest baseline Lp(a) levels. Similarly, alirocumab (in ODYSSEY OUTCOMES – Evaluation of Cardiovascular Outcomes After and Acute Coronary Syndrome During Treatment With Alirocumab) had increased MACE reduction in individuals with higher baseline Lp(a) levels. While these results are encouraging, the impact of PCSK9 inhibitors on Lp(a) is fairly modest, and additional analysis is needed before PCSK9-inhibitors can be recommended as Lp(a)-targeted therapy.
Apheresis may be considered for select patients. It is highly effective at lowering Lp(a) levels but a costly and cumbersome procedure that may be difficult to obtain insurance coverage for. It is reserved for only the most refractory patients and should be pursued after optimally controlling known risk factors with proven therapies. In Germany, apheresis appeared to yield a 70% reduction in MACE in patients with recurrent ASCVD events with elevated Lp(a) regardless of LDL-C levels.
Importantly, a number of commonly used preventive strategies are ineffective. Most notably, lifestyle changes, including diet and exercise, do not reduce Lp(a). Early data on statins showed mixed results with some studies suggesting they may even lead to increases in Lp(a). However more contemporary studies show that statin therapy in itself does affect Lp(a) levels; though importantly, those who continue to have high Lp(a) levels on a statin are at increased ASCVD risk even if LDL-C is improved. Hormone replacement therapy reduces Lp(a), but its use is limited by the excess increase in adverse events. Niacin reduces Lp(a) 23% but did not improve ASCVD outcomes based on the AIM HIGH (Niacin Plus Statin to Prevent Vascular Events) and HPS2 THRIVE (Treatment of HDL to Reduce the Incidence of Vascular Events) studies. While tolerated by some, niacin is also associated with significant side effects. Furthermore, its efficacy is limited in those who may benefit the most (small isoform size and highest baseline Lp[a] levels).
Based on the available data, the authors recommend initiating a moderate- to high-intensity statin therapy in adults aged 40-75 years with a 10-year ASCVD risk of 7.5% to ≤20% with a Lp(a) ≥100 nmol/L. As is already commonly done, high risk patients with LDL-C ≥70 mg/dL (non-HDL-C ≥100 mg/dL) and a Lp(a) ≥100 nmol/L on maximally tolerated statin should be considered for more intensive therapies (ezetimibe and PCSK9 inhibitors) to lower LDL-C.
Currently, novel therapies are being studied that selectively target Lp(a). A phase 2 trial of AKCEA apo(a)-LRx, an apo(a) antisense oligonucleotide, reduced Lp(a) up to 80%. A phase 3 study is being planned. Additionally, an oxPL antibody that binds and inactivates the pro-osteogenic activity of Lp(a) has promising in vitro data. These therapies, while promising, require additional research prior to becoming mainstream therapies.
Conclusion
Lipoprotein(a) represents an exciting new biomarker in the field of lipidology and preventive cardiology. Elevated Lp(a) is causally implicated in ASCVD, and testing in specific patients may help to tailor the appropriate intensity of preventive measures. However, because of the lack of standardization and heterogeneity of the available data, optimal cut-offs remain a source of intense debate. The current focus of clinical care is on traditional risk factor control in patients with high Lp(a) as targeted treatment options are limited. Novel Lp(a)-targeting therapies are actively being investigated, and if successful, they could become an important component of primary and secondary prevention.
Clinical Topics: Acute Coronary Syndromes, Anticoagulation Management, Cardiovascular Care Team, Diabetes and Cardiometabolic Disease, Dyslipidemia, Prevention, Valvular Heart Disease, ACS and Cardiac Biomarkers, Anticoagulation Management and ACS, Advanced Lipid Testing, Homozygous Familial Hypercholesterolemia, Lipid Metabolism, Nonstatins, Novel Agents, Primary Hyperlipidemia, Statins, Diet
Keywords: Dyslipidemias, Acute Coronary Syndrome, American Heart Association, Antibodies, Monoclonal, Aortic Valve Stenosis, Apolipoproteins A, Apolipoproteins B, Blood Component Removal, Brain Ischemia, Biomarkers, Cardiovascular Diseases, Cholesterol, Chemistry, Clinical, Constriction, Pathologic, Comorbidity, Diet, Disulfides, Decision Making, Ethnic Groups, Fibrinolysis, Genome-Wide Association Study, Genetic Variation, Hormone Replacement Therapy, Hydroxymethylglutaryl-CoA Reductase Inhibitors, Hypercholesterolemia, Hyperlipoproteinemia Type II, Inflammation, Inheritance Patterns, Life Style, Insurance Coverage, Lipoprotein(a), Lipoproteins, LDL, Niacin, Oligonucleotides, Antisense, Particle Size, Phospholipids, Plasminogen, Prospective Studies, Protein Isoforms, Random Allocation, Risk Factors, Risk Assessment, Secondary Prevention, Stroke, Thrombosis, World Health Organization, Subtilisins
< Back to Listings