Surgical Management of Severe FMR: What Can COAPT and MITRA-FR Teach Us?
The surgical management of severe functional mitral regurgitation (FMR) has long been a challenge for surgeons. Despite evidence showing acceptable short-term outcomes and improvement in heart failure (HF) symptoms with mitral valve (MV) surgery for severe FMR, no study has demonstrated a mortality benefit when compared with patients treated with medical management.1,2 Unlike degenerative or primary mitral regurgitation, where correction of leaflet pathology has excellent short- and long-term outcomes, surgical management of FMR, the pathophysiology of which lies in dysfunction of the left ventricle (LV), has been less successful.3
With the equivocal results of surgical therapy, transcatheter options have been developed for the management of FMR. The most commonly used transcatheter option includes the MitraClip (Abbott; Abbott Park, IL) MV repair system. Two recent randomized trials were performed, on two separate continents, to compare the use of MitraClip versus medical therapy in the management of FMR. The US COAPT trial (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation) was the first study to show a mortality benefit with intervention to treat FMR; its results differed from the European MITRA-FR trial (Percutaneous Repair With the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation), which failed to detect any difference with treatment.4,5 Each trial randomized patients with chronic HF, reduced ejection fraction (EF), and severe secondary mitral regurgitation who were not eligible for MV surgery to receive a MitraClip versus medical therapy. Both trials reported good rates of contemporary medical therapy during the follow-up period, but only the COAPT trial required strict guideline-directed medical therapy prior to randomization.
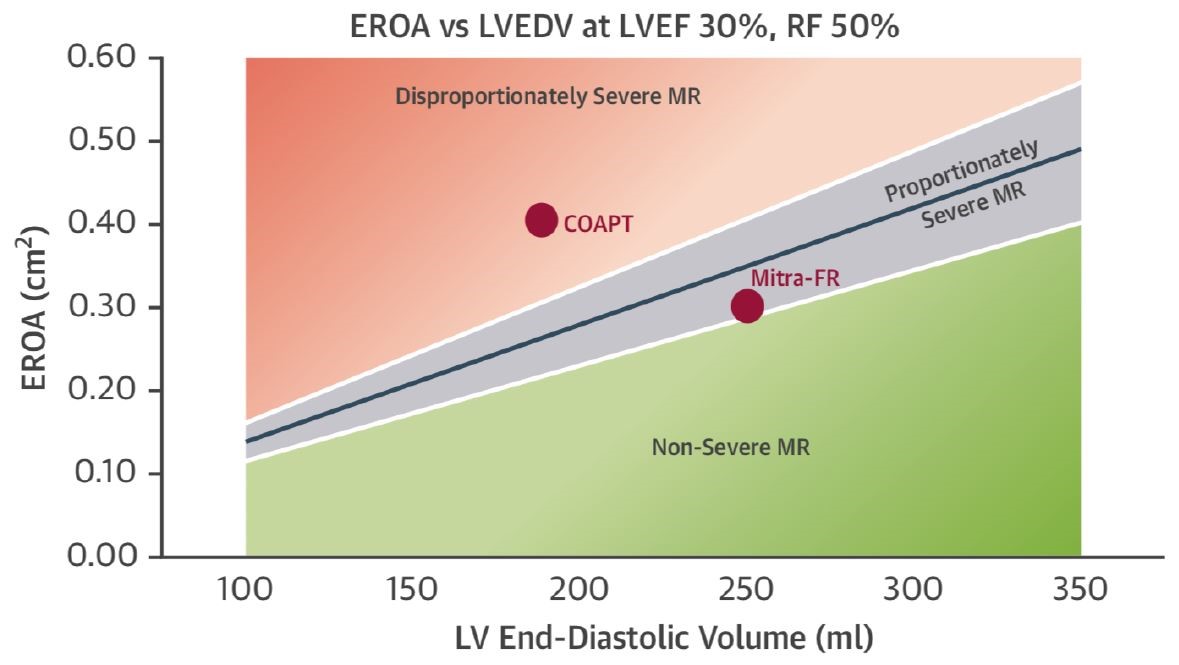
The disparity in results between the COAPT and MITRA-FR studies may significantly improve our understanding of which patients benefit from this intervention to correct FMR. LVEF did not differ between trials (31% in COAPT; 33% in MITRA-FR), but COAPT included patients with EF ≥20%, and MITRA-FR included patients with EF ≥15-40%. Those in the MITRA-FR trial had larger LV volumes (LV end diastolic volume 252 mL vs. 192 mL) and less mitral regurgitation (effective regurgitant orifice area [EROA] 31 mm2 vs. 41 mm2) than patients in COAPT, which excluded patients with very large LV (LV end-systolic diameter ≤7 0mm). The relationship between pre-operative LV size and the degree of reverse remodeling is not new and has been shown in surgical series.6 Grayburn and colleagues elegantly describe a new conceptual framework by which to consider FMR and the role of proportionate versus disproportionate mitral regurgitation and use this framework to rationalize the differing results of MITRA-FR and COAPT (Figure 1).7 Mitral regurgitation is "expected" to increase in proportion to increasing LV size as a result of leaflet tethering and annular dilatation. Indeed, the EROA is dependent on the LV size and function for any given regurgitant fraction; its use in isolation without the other variables is thus limited.8 The EROA threshold required to achieve a regurgitant fraction of >50% (or severe mitral regurgitation) increases with increasing LV size or decreasing function. In proportionate mitral regurgitation, the estimated degree of mitral regurgitation is proportionate to the size of the LV. In disproportionate mitral regurgitation, the degree of mitral regurgitation is unexpectedly large compared with the LV size. This concept was validated, but Bartko and colleagues noted that survival was worst in those with disproportionate (i.e., relatively severe) mitral regurgitation compared with proportionate (i.e., relatively non-severe) mitral regurgitation.9 The MITRA-FR study enrolled patients with relatively large LV size with only modest mitral regurgitation. Those in COAPT had a greater degree of mitral regurgitation compared with the LV size; those in COAPT who did not benefit had relatively modest mitral regurgitation (<30 mm2) with larger LV (>96 mL/m2). Based on these results, it appears that patients who are likely to realize benefit from MV intervention are those with the greatest amount of mitral regurgitation with respect to LV size and function. These patients will have the least amount of LV remodeling (small LV) with the greatest amount of mitral regurgitation.
Figure 1: Disproportionate Versus Proportionate FMR6
The requirement for COAPT patients to have maximally tolerated guideline-directed medical therapy (including cardiac resynchronization therapy) prior to enrollment may have assisted in selecting patients in whom the LV size was already optimized, yet still had severe mitral regurgitation (thereby excluding those with proportionate mitral regurgitation). This was not a requirement of the MITRA-FR trial, and how this translates to real world practice is unclear.
What have we learned from the surgical literature? The only attempt at a randomized trial comparing surgery versus medical therapy for severe FMR was terminated due to difficulties in recruitment: SMMART-HF (Effectiveness of Surgical Mitral Valve Repair Versus Medical Treatment for People With Significant Mitral Regurgitation and Non-ischemic Congestive Heart Failure). A retrospective analysis compared patients who underwent MV annuloplasty with patients who were eligible for, but did not undergo, annuloplasty.1 There was no difference in the primary endpoint of death, LV assist device, or transplant between groups at approximately 3 years follow-up. Treatment with angiotensin-converting enzyme inhibitor and beta-blockers, but not MV repair, were independently protective of the primary endpoint.
Several studies have demonstrated symptomatic and echocardiographic benefit of MV repair.2,10,11 In the Cardiothoracic Surgical Trials Network (CTSN) trial comparing MV repair or replacement for severe FMR (effective regurgitant orifice = 40 mm2; LV end systolic volume index = ~63 mL/m2; estimated LV end diastolic volume = ~200 mL: disproportionate mitral regurgitation), there was no difference in early mortality (1.6% for repair vs. 4.0% for replacement; p = 0.26) or LV remodeling between MV repair or replacement.12-14 Although there was no difference in mortality at 2 years between groups (19% for MV repair vs. 23.4% for MV replacement), those with MV replacement had significantly less recurrent mitral regurgitation at 2 years than those who were replaced (59% vs. 4%). This resulted in only a trend to increased symptomatic improvement in the replacement group. Not surprisingly, those without recurrent mitral regurgitation (regardless of treatment) had significantly fewer symptoms than those with mitral regurgitation. In the repair group, those without recurrent mitral regurgitation had significantly greater reverse remodeling at follow-up, but there was no difference in baseline characteristics (including LV size) to predict those with and without recurrence. The mechanism of mitral regurgitation recurrence is not only posterior leaflet tethering,15 but also factors beyond LV size alone, such as impairment of lateral shortening size between papillary muscles.16 Some have suggested technical failure (a failure to address laterally displaced papillary muscles) as a potential cause of the high recurrence rate in the MV repair group.14 The results indicate that patients have the best LV remodeling if they have MV repair without recurrent mitral regurgitation; however, the high rate of recurrence after MV repair has encouraged the majority of surgeons to replace the valve for severe ischemic mitral regurgitation. Interestingly, 9 of 25 patients with moderate or severe mitral regurgitation in the repair group at 6 months had only mild mitral regurgitation at 12 months. This suggests the dynamic nature of the mitral regurgitation and the potential to improve it with medical therapy.15
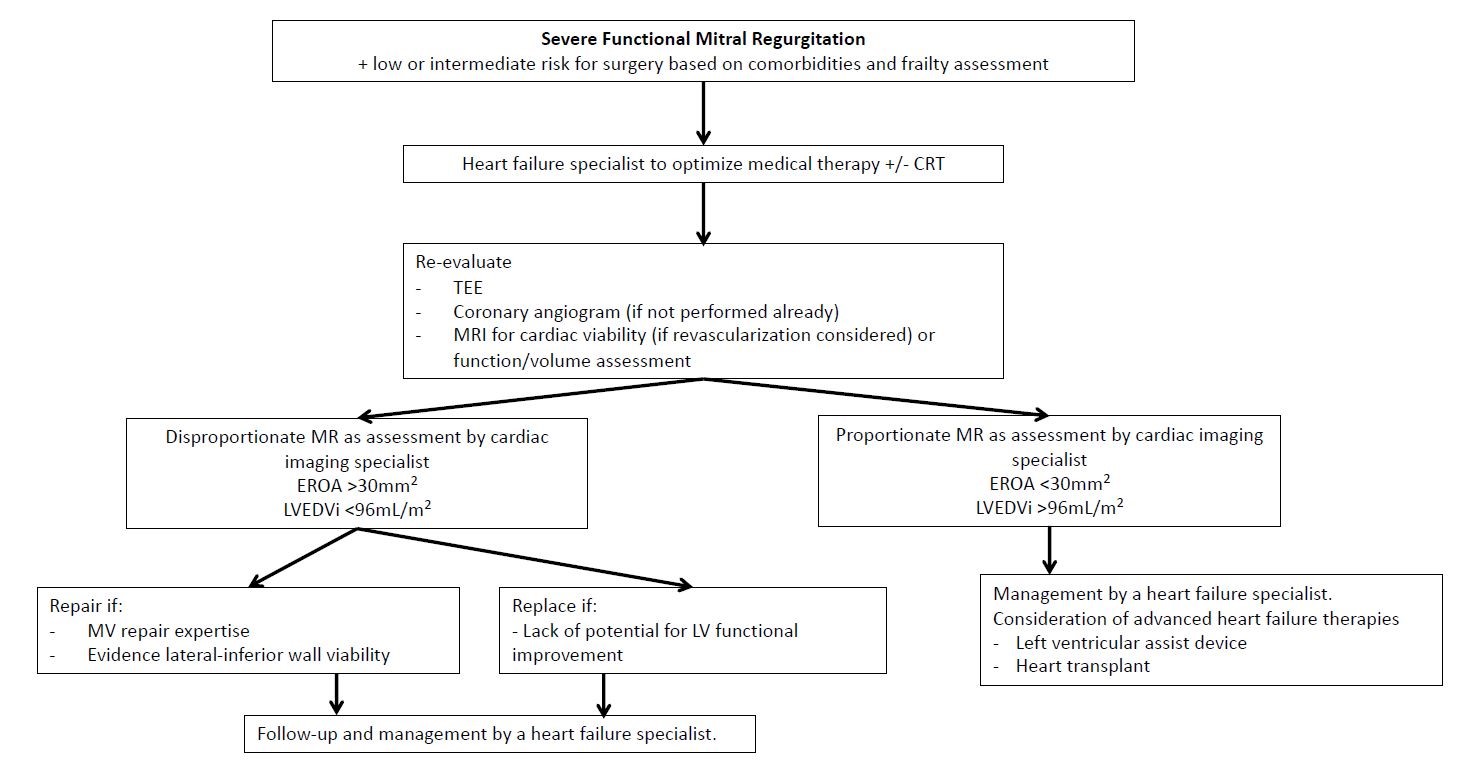
How can the results of the transcatheter trials be applied to the surgical treatment of mitral regurgitation? The COAPT and MITRA-FR trials have significantly filled the knowledge gap for the medical and interventional treatment of FMR. The very complex and dynamic aspects of FMR represent an interplay between the MV apparatus and the LV; thus, a strategy to improve LV function is fundamental to the solution. The importance of an LV-focused approach is highlighted in another CTSN trial of surgical treatment of moderate ischemic mitral regurgitation, where 67.7% of patients randomized to coronary artery bypass grafting alone (compared with coronary artery bypass grafting plus MV repair) had improvement in mitral regurgitation at 2 years.17 The COAPT and MITRA-FR trials have shown us the importance of pre- and post-operative medical therapy directed by a HF specialist and other strategies to improve LV function, including cardiac resynchronization therapy or revascularization. Figure 2 details our approach to patients with FMR. A team approach, including HF specialists to maximize medical therapy and cardiac imaging specialists to assist in image analysis, will assist the interventional cardiologist and cardiac surgeon in selecting from the variety of treatment options available for this patient population. Only patients with mitral regurgitation out of proportion to that expected of the LV dysfunction should be considered for surgery. Better understanding and application of mitral repair techniques that include the subvalvular apparatus may improve the durability of our repairs, and this may ultimately be the best treatment.
Figure 2: A Framework for our Treatment of Patients With FMR
References
- Wu AH, Aaronson KD, Bolling SF, Pagani FD, Welch K, Koelling TM. Impact of mitral valve annuloplasty on mortality risk in patients with mitral regurgitation and left ventricular systolic dysfunction. J Am Coll Cardiol 2005;45:381-7.
- Bolling SF, Pagani FD, Deeb GM, Bach DS. Intermediate-term outcome of mitral reconstruction in cardiomyopathy. J Thorac Cardiovasc Surg 1998;115:381-6.
- Gammie JS, Chikwe J, Badhwar V, et al. Isolated Mitral Valve Surgery: The Society of Thoracic Surgeons Adult Cardiac Surgery Database Analysis. Ann Thorac Surg 2018;106:716-27.
- Stone GW, Lindenfeld J, Abraham WT, et al. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med 2018;379:2307-18.
- Obadia JF, Messika-Zeitoun D, Leurent G, et al. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N Engl J Med 2018;379:2297-306.
- Braun J, Bax JJ, Versteegh MI, et al. Preoperative left ventricular dimensions predict reverse remodeling following restrictive mitral annuloplasty in ischemic mitral regurgitation. Eur J Cardiothorac Surg 2005;27:847-53.
- Grayburn PA, Sannino A, Packer M. Proportionate and Disproportionate Functional Mitral Regurgitation: A New Conceptual Framework That Reconciles the Results of the MITRA-FR and COAPT Trials. JACC Cardiovasc Imaging 2019;12:353-62.
- Grayburn PA, Carabello B, Hung J, et al. Defining "severe" secondary mitral regurgitation: emphasizing an integrated approach. J Am Coll Cardiol 2014;64:2792-801.
- Bartko PE, Heitzinger G, Arfsten H, et al. Disproportionate Functional Mitral Regurgitation: Advancing a Conceptual Framework to Clinical Practice. JACC Cardiovasc Imaging 2019;12:2088-90.
- De Bonis M, Taramasso M, Verzini A, et al. Long-term results of mitral repair for functional mitral regurgitation in idiopathic dilated cardiomyopathy. Eur J Cardiothorac Surg 2012;42:640-6.
- Braun J, van de Veire NR, Klautz RJ, et al. Restrictive mitral annuloplasty cures ischemic mitral regurgitation and heart failure. Ann Thorac Surg 2008;85:430-6.
- Acker MA, Parides MK, Perrault LP, et al. Mitral-valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med 2014;370:23-32.
- Goldstein D, Moskowitz AJ, Gelijns AC, et al. Two-Year Outcomes of Surgical Treatment of Severe Ischemic Mitral Regurgitation. N Engl J Med 2016;374:344-53.
- Packer M, Grayburn PA. Contrasting Effects of Pharmacological, Procedural, and Surgical Interventions on Proportionate and Disproportionate Functional Mitral Regurgitation in Chronic Heart Failure. Circulation 2019;140:779-89.
- Kron IL, Hung J, Overbey JR, et al. Predicting recurrent mitral regurgitation after mitral valve repair for severe ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2015;149:752-61.e1.
- Kalra K, Wang Q, McIver BV, et al. Temporal changes in interpapillary muscle dynamics as an active indicator of mitral valve and left ventricular interaction in ischemic mitral regurgitation. J Am Coll Cardiol 2014;64:1867-79.
- Michler RE, Smith PK, Parides MK, et al. Two-Year Outcomes of Surgical Treatment of Moderate Ischemic Mitral Regurgitation. N Engl J Med 2016;374:1932-41.
Keywords: Mitral Valve, Mitral Valve Insufficiency, Heart Ventricles, Stroke Volume, Follow-Up Studies, Random Allocation, Heart Failure, Cardiac Surgical Procedures, Aneurysm, Surgeons, Outcome Assessment, Health Care, Papillary Muscles, Cardiac Resynchronization Therapy, Retrospective Studies, Specialization, Adrenergic beta-Antagonists, Echocardiography, Angiotensin-Converting Enzyme Inhibitors, Coronary Artery Bypass, Dilatation
< Back to Listings