The Role of Vericiguat in the Expanding Realm of Heart Failure Pharmacotherapy: An Overview of the VICTORIA Trial
Quick Takes
- Antagonistic effects of the heart failure syndrome include endothelial dysfunction and reactive oxygen species generation, which have not been historically targeted in heart failure guideline directed therapy.
- In a large, multicenter, randomized, placebo-controlled trial among patients with chronic heart failure and left ventricular ejection fraction <45%, the addition of vericiguat (an oral guanylate cyclase stimulator) to guideline-based medical therapy resulted in a small but statistically significant reduction in a composite clinical endpoint of cardiovascular death or first hospitalization for heart failure.
- Patients with age less than 75 years, chronic renal insufficiency, LV ejection fraction <45%, and NYHA functional class III or IV appear to receive further benefit. Further investigation is needed to elucidate the role of vericiguat amongst available evidence-based therapies and target populations.
After decades of a relatively static heart failure with reduced ejection fraction (HFrEF) regimen with beta-blockers and renin-angiotensin-aldosterone antagonists, there have been numerous trials demonstrating the incremental benefit of novel pharmacotherapeutics. The PARADIGM trial shifted the HFrEF treatment framework, as sacubitril-valsartan reduced mortality and HF hospitalizations compared to enalapril.1 Recently, the DAPA-HF trial showed that the addition of dapagliflozin (a SGLT2 inhibitor) to standard HF guideline-directed medical therapy in NYHA class II-IV HFrEF patients conferred protection from worsening HF or cardiovascular mortality, regardless of the presence or absence of diabetes.2 Based on this study, the US Food and Drug Administration recently approved dapagliflozin for treatment of HFrEF.3 Within this context of progression in treatment options for HFrEF, the VICTORIA trial presents another pharmacologic option for chronic HFrEF with vericiguat.4
Vericiguat is a novel pharmacotherapy that modulates endothelial dysfunction, an often underrecognized component of the HF syndrome. The vascular endothelium normally generates nitric oxide (NO) which stimulates soluble guanylate cyclase (sGC) mediated cyclic guanosine monophosphate (cGMP) production. Similarly, the endocardial endothelium is sensitive to NO and regulates contractility and diastolic function by raising intracellular cGMP. This process becomes dysregulated in heart failure, leading to NO-sGC-cGMP insufficiency and causes impairment in diastolic relaxation and microvascular dysfunction. Historically, nitrates, in combination with hydralazine, have been shown to reduce mortality in HFrEF,5 although this benefit has only been shown in the Black population.6 Vericiguat is an sGC activator, jointly developed by Merck and Bayer, which modulates sGC and enhances the effect of NO to increase cGMP activity.7 This could potentially be a reason for the efficacy of vericiguat in a broader HFrEF population. Early studies have demonstrated safety and efficacy of stimulating sGC in patients with HFrEF.8,9
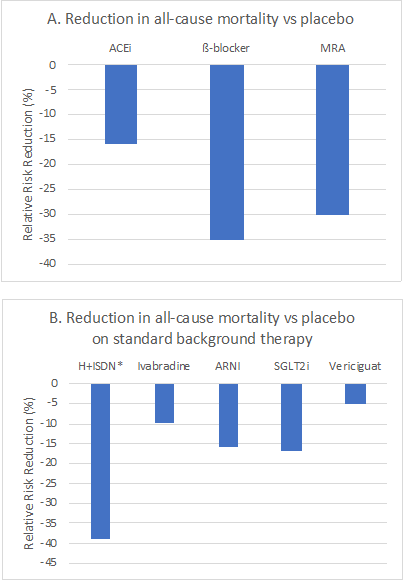
The VICTORIA trial investigated the effects of vericiguat compared with placebo in 5,050 patients with chronic HF (NYHA class II-IV), left ventricular ejection fraction (LVEF) <45%, not receiving nitrates, recent HF decompensation treated with hospitalization or outpatient intravenous (IV) diuretics, and elevated brain natriuretic peptide (BNP) levels.10 Over a median follow-up of 10.8 months, there was a 10% reduction in the primary endpoint of cardiovascular death or HF hospitalization (hazard ratio 0.90, 95% CI 0.83-0.98, p = 0.02). The target dose was achieved in 89% of these patients, and there was no difference in syncope or hypotension rates. There was a signal for greater benefit in patients with age <75 years, LVEF <40%, renal insufficiency, patients not on an angiotensin receptor neprilysin inhibitor (ARNI), and NYHA class III or IV.
This trial demonstrates that a novel sGC modulator is safe, well tolerated, and improves HF outcomes without requiring midday dosing or laboratory testing. The inclusion criteria were more stringent than the PARADIGM-HF1 and DAPA-HF2 trials. VICTORIA had a BNP or NT-proBNP requirement two-fold higher than that of PARADIGM or DAPA-HF, and it required enrollment within 6 months of HF hospitalization or, without hospitalization, within 3 months of IV diuretic therapy. In addition, the patients enrolled were on excellent background therapy with >90% on beta blockers and either ACEi, ARB, or ARNI. More than 40% of the patients had stage 3 chronic kidney disease (CKD), thus conferring cardiorenal benefits in this high-risk population. The study primarily enrolled Caucasian (64.1%) and Asian (22.4%) participants. With Black patients (4.9%) being underrepresented and patients on nitrates being excluded, there are subpopulations that remain unexplored.
Questions remain about the optimal treatment strategy for a patient with HFrEF. In this new era of medical therapies including ARNI, sodium-glucose cotransporter-2 (SGLT2) inhibitors, and sGC modulators, the potential combinations of therapies are now expansive, and we need further research to understand how to best prioritize these new therapies. One may utilize the familiar workhorse agents of an ACEi/ARB, beta blocker, and mineralocorticoid receptor antagonists (MRA), but recognizing the differences and advantages of each of these novel agents will allow for the ability to personalize specific regimens based on underlying risks and comorbidities. For instance, a recently hospitalized patient treated with a regimen of ARNI, carvedilol, and spironolactone may benefit from the addition of vericiguat. Likewise, in a diabetic patient with CKD and HFrEF, a SGLTi would be the first preference for incremental therapy. Let us not forget that traditional HF therapies remain underutilized.11 A Get with The Guidelines-HF registry analysis suggests there may be room for increased uptake of traditional HFrEF guideline-directed medical therapy (GDMT) before adding new drugs based on low adherence to existing GDMT after 1 year (53% to beta blockers, 48% for ACE/ARB, 46% MRA).
Achieving the "quintuple therapy" of RAAS inhibition (ACEi/ARB/ARNI and MRA), beta blocker, SGLTi, and an sGC modulator could be the ideal regimen, but it remains to be seen how one accomplishes this through the ritual of outpatient medication titration and adherence. Legacy therapies such as hydralazine-nitrates and digoxin need to be weighed against contemporary HFrEF. In addition, cost considerations must be addressed, as insurance denials and prior authorizations will ultimately dictate both patient and physician preferences. Additional emerging treatments such as omecamtiv12 may complicate this process further. While we await further guidance in this era of novel HF therapies and the real-world experience, we can garner the experience from existing trials to develop practice patterns to aid our patients. As we continue to digest the available evidence and better understand these therapies, it will be crucial to explore remaining gaps in knowledge to provide optimal care to our HFrEF cohorts.
Figure 1: Reduction in all-cause mortality with studied pharmacotherapies for heart failure with reduced ejection fraction as reported in landmark trials.
*A-HeFT investigated hydralazine/nitrates in self-identified Black patients on background therapy of angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB) and beta-blockers.
References
- McMurray JJV, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993-1004.
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995-2008.
- FDA approves new treatment for a type of heart failure (FDA.gov website). 2020. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-type-heart-failure. Accessed 04/30/2020.
- Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020;382:1883-93.
- Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med 1986;314:1547-52.
- Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in Blacks with heart failure. N Engl J Med 2004;351:2049-57.
- Gheorghiade M, Marti CN, Sabbah HN, et al. Soluble guanylate cyclase: a potential therapeutic target for heart failure. Heart Fail Rev 2013;18:123-34.
- Bonderman D, Ghio S, Felix SB, et al. Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation 2013;128:502-11.
- Gheorghiade M, Greene SJ, Butler J, et al. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: the SOCRATES-REDUCED randomized trial. JAMA 2015;314:2251-62.
- Armstrong PW, Roessig L, Patel MJ, et al. A multicenter, randomized, double-blind, placebo-controlled trial of the efficacy and safety of the oral soluble guanylate cyclase stimulator: the VICTORIA Trial. JACC Heart Fail 2018;6:96-104.
- Chang LL, Xu H, DeVore AD, et al. Timing of postdischarge follow-up and medication adherence among patients with heart failure. J Am Heart Assoc 2018;7:e007998.
- Teerlink JR, Diaz R, Felker GM, et al. Omecamtiv mecarbil in chronic heart failure with reduced ejection fraction: rationale and design of GALACTIC-HF. JACC Heart Fail 2020;8:329-40.
Clinical Topics: Dyslipidemia, Heart Failure and Cardiomyopathies, Lipid Metabolism, Novel Agents, Acute Heart Failure, Heart Failure and Cardiac Biomarkers
Keywords: Heart Failure, acc20, ACC Annual Scientific Session, Mineralocorticoid Receptor Antagonists, Spironolactone, Stroke Volume, Nitric Oxide, Natriuretic Peptide, Brain, Renin, Nitrates, Digoxin
< Back to Listings