COVID-19 and Use of Drugs Targeting the Renin-Angiotensin-System
Cardiovascular disease, kidney disease, and hypertension are frequently treated with angiotensin-converting-enzyme inhibitors (ACEIs) and angiotensin-receptor blockers (ARBs), and they are also commonly associated with increased severity and mortality from the coronavirus disease, COVID-19. Diabetes is a common co-morbidity associated with each of these disorders. Some have speculated that treatment with ACEIs and ARBs may be at least in part responsible for the increased severity and mortality from COVID-19 in those with these co-morbidities.1 A recent article in MedPage reviewed the results of three studies that support the continued use of these agents in COVID-19 patients.2 In this article, we review the results and conclusions of these and other studies regarding ACEI and ARB use in this COVID-19 era.
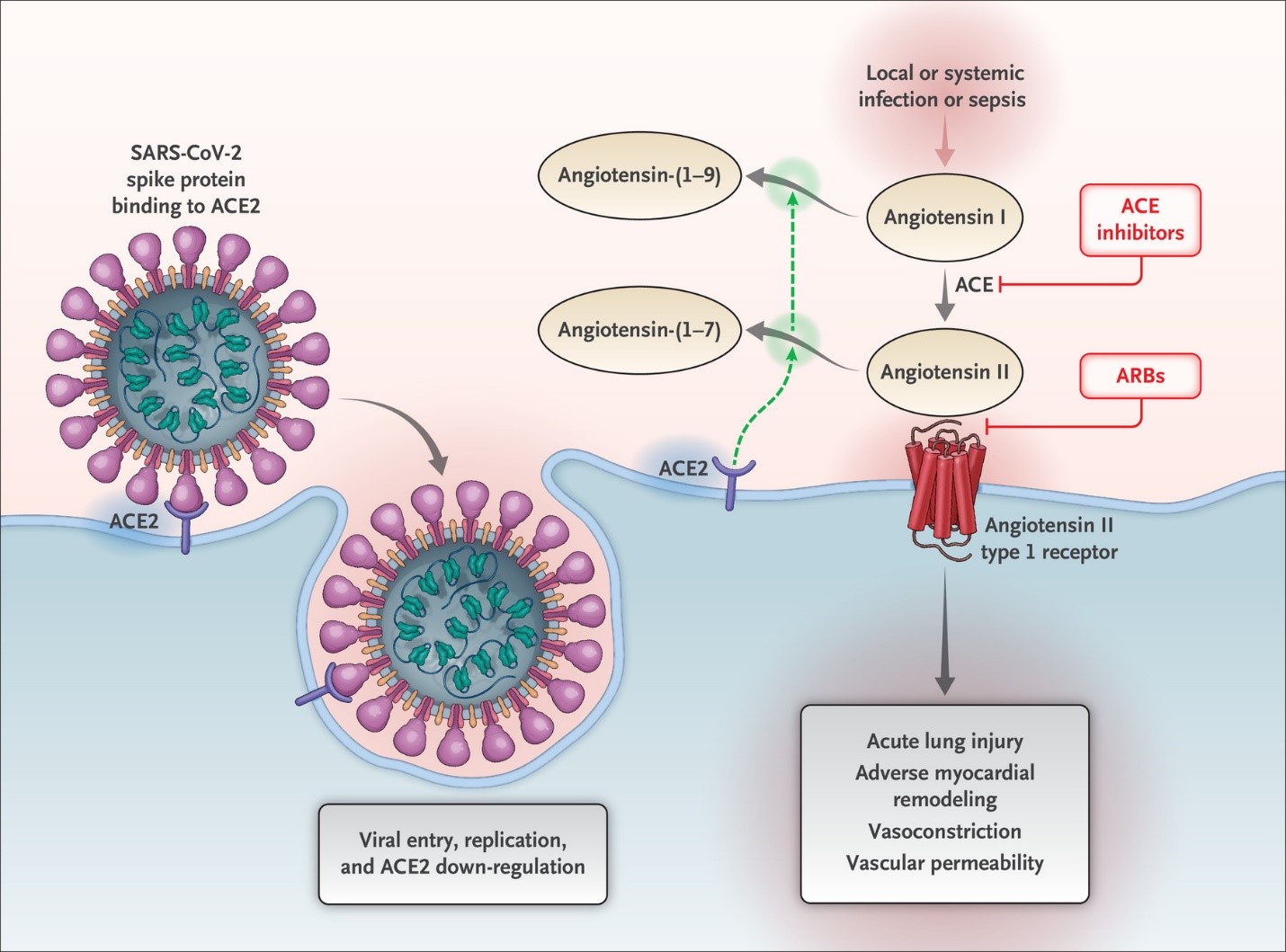
COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which enters human cells by binding its spike protein (S) to the angiotensin converting enzyme-2 (ACE2) cell receptor via a protease dependent mechanism (Figure 1).3,4 These ACE2 receptors are present in the epithelium of the lung (the site of SARS-CoV-2 entry) as well as the proximal tubule of the kidney, myocardium, blood vessels, ileum, esophagus, and bladder.5 While ACEIs and ARBs have no direct interaction with the ACE2 receptor, some but not all previous work has shown that they may result in an up-titration of these receptors.6-8 In addition, glucose lower agents such as the sodium glucose cotransporter 2 inhibitors and thiazolidines in the treatment of diabetes mellitus also elevate ACE2 receptors. ACE2 receptors, by degrading angiotensin II (1-9) into angiotensin 1-7, reduce the vasoconstriction, sodium retention, and fibrosis resulting from angiotensin II which may serve a protective role against COVID-19 (Figure 1).4,9 Alternatively, given that ACE2 receptors might facilitate coronavirus entry, multiplication, spread, and pathogenesis, there is concern that patients on ACEI/ARB may be more susceptible to COVID-19 infection and/or at greater risk for severe illness or death once infected. A recent report in patients with heart failure found no association between ACEI/ARB use and circulating plasma concentrations of ACE2.10 Thus, it remains unclear what association, if any, exists between ACEI/ARB treatment and ACE2 receptor activity.
Figure 1: Schematic depicting the mechanism of ACE2 (the site of SARS-CoV-2 entry) and ACEi/ARB therapy
Clinical studies to examine the risk associated with ACEI and ARB treatment in patients with COVID-19 are increasing but remain limited, and data on the individual risk of these agents are even more limited. The MedPage article reviewed the results of three recent observational studies examining the effect of ACEI/ARB drugs on the risk of COVID-19 outcomes.11-13 In the first, a retrospective cohort that examined 12,594 patients in New York City, 46.8% of whom tested positive for COVID-19, Reynolds et al. found no association between ACEI or ARB use and COVID-19 positivity or infection-related severe illness or mortality.11 In another, Mancia and colleagues compared 6,722 cases in an Italian cohort to 30,759 age and sex-matched controls and reported that cases were 10% more likely to be on ACEI or ARB treatment compared to controls.12 However, this difference disappeared after adjusting for the higher prevalence of cardiovascular disease among cases. The third study, by Mehra et al., looked at survivorship versus fatalities among 8,910 hospitalized patients that tested positive for COVID-19 across 169 hospitals in Asia, Europe, and North America and found that the odds of death due to COVID-19 were not significantly higher for those on ACEI or ARB therapy.13 However, this report was subsequently withdrawn by the journal, and the results may likely be falsified. Two additional studies with more recent data reported results that supported the safety of these agents in COVID infected patients. Zhang and colleagues studied 3,611 COVID positive patients hospitalized in Hubei, China.14 They assessed in-hospital use of ACEI or ARB therapy without accounting for ACEI/ARB use prior to hospitalization and reported a lower risk of all-cause mortality in COVID-19 patients. Finally, Fosbøl et al. reported a country-wide study of all COVID-19 infected patients (n=4,480) between February 1 and May 4, 2020 from the Danish registry.15 The study also included a nested case control comparison of ACEI/ARB versus other antihypertensive drug use in the 6 months prior to diagnosis for incidence of COVID-19 diagnosis. They found no significant association between ACEI/ARB use and severe COVID-19 outcomes, mortality, or incident infection.
ACEI and ARB use have also been hypothesized to increase the risk for COVID-19 infection in individuals with diabetes mellitus.16 However, the data on the risk of COVID-19 in patients with diabetes and the effect of ACEI/ARB use on outcomes in this population is extremely limited. The prevalence of patients with diabetes in the above studies ranged from only approximately 10-24% with less than half on an ACEI or ARB. A systematic review of 14 relatively small retrospective studies found a higher prevalence of diabetes in patients infected with COVID-19.17 Two studies that examined cardiac-specific enzymes reported higher rates of cardiac injury in COVID-19 patients with comorbid diabetes than those without diabetes.18,19 However, none of these observational studies were able to adequately adjust for the confounding since those on ACEI or ARB tended to be older and more likely to have hypertension, chronic kidney disease (CKD), obesity, and other cardiovascular diseases.
Multiple US and international cardiovascular societies have issued statements after review of the available data.20 Their consensus recommendation has uniformly been to continue ACEI or ARB therapy and to not hesitate to use these drugs in patients where there is a specific indication for their use (e.g., left ventricular (LV) dysfunction, CKD, secondary stroke prevention). While it is important to acknowledge the limitations of the observational and retrospective studies thus far in eliminating the possibility of confounding, there has not been evidence of an increased risk of morbidity or mortality in several large trials with different study designs (including both cohort and case-control). This provides some reassurance for the clinician facing a dilemma of whether to continue their patient on an ACEI or ARB. Given that COVID-19 appears to have a greater prevalence and lethality in those with underlying cardiovascular conditions, especially hypertension,21 adequate treatment of a patient with these disorders with an ACEI/ARB or other antihypertensive agents is preferred over the alternative of inadequate disease management.
Despite the Fosbøl study,15 the question of whether the initiation of an ACEI or ARB may place the uninfected patient at increased risk for COVID-19 or present a worse outcome if they become infected, particularly in those without a specific indication for these agents, remains unresolved. The definitive answer must await the results of prospective randomized controlled outcome trials to document their benefit in COVID infected patients or at least a lack of increased risk in patients who are COVID naive. There are several prospective, interventional trials currently underway to examine the use of ACEI/ARB on outcomes in patients with COVID-19. These include randomized clinical trials where the ACEI or ARB are discontinued in a COVID-19 infected patient already on one of these agents.22 Two others involve the randomized use of losartan in COVID-19 infected patients for outcomes in both outpatient and inpatient settings (ClinicalTrials.gov Identifiers: NCT04312009, NCT04311177).23,24 Another involves switching patients already on ACEI or ARB to a calcium channel blocker or thiazide-type diuretic (ClinicalTrials.gov Identifier: NCT04330300).25
In the absence of more definitive data, there remains significant uncertainty regarding the effect of the initiation of an ACEI or ARB in patients without comorbidities denoting a compelling indication for their use. Due to this uncertainty and until more data on the risk/benefit of ACEI/ARB use in patients with or at risk for COVID-19 becomes available, we recommend using the other first-line agents (thiazide-type diuretics [chlorthalidone preferred] and calcium channel blockers) recommended by current US guidelines in preference to these agents.26 Once a vaccine or effective treatment for COVID-19 has emerged, any of the first-line antihypertensive medications recommended in the US guidelines would again become acceptable.
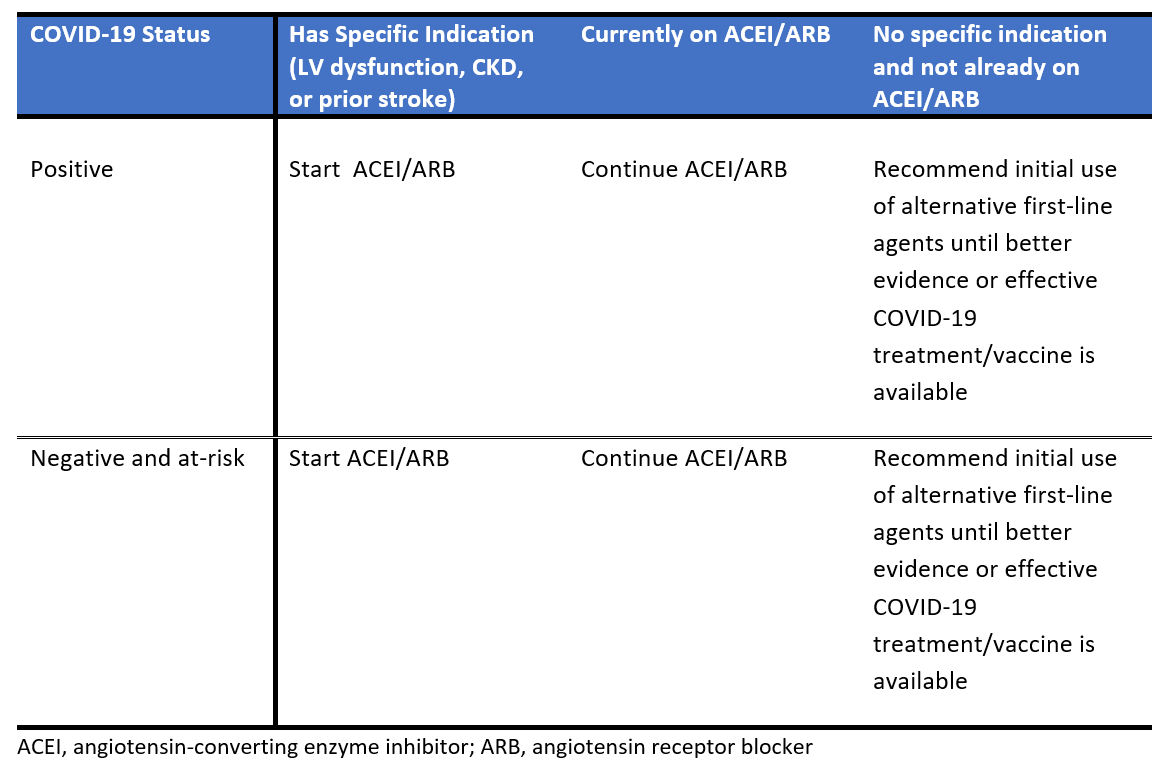
Table 1 summarizes our recommendations regarding ACEI/ARB use in the relevant populations. The available evidence currently supports the recommendation outlined here, and that of most professional societies continue the use of ACEI and ARB therapy for those already taking these agents for specific indications. They should be initiated in any patient with specific indications for these agents or when additional agents are needed for blood pressure (BP) control. For those patients who are already on ACEI/ARB therapy at the time of COVID-19 infection, these medications should not be discontinued. In the absence of clinical data on risk/benefit or until we have control of COVID-19 (effective treatment or vaccine), we recommend that these agents be used only after other first line-agents for hypertension, except in those with another indication to be on an ACEI/ARB medication (e.g., LV dysfunction, CKD especially with proteinuria, secondary stroke protection).
Table 1: Summary of recommendations regarding ACEI/ARB use in the outpatient setting during COVID-19 outbreak
References
- Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020;8:e21.
- Phend C. Hypertension drugs appear safe in COVID-19 (MedPage Today website). 2020. Available at: https://www.medpagetoday.com/infectiousdisease/covid19/86277. Accessed 06/20/2020.
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271-80.e8.
- Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with COVID19. N Engl J Med 2020; 382:1653-9.
- Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 2020;14:185-92.
- Zisman LS. ACE and ACE2: a tale of two enzymes. Eur Heart J 2005;26:322-4.
- Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005;111:2605–10.
- Soler MJ, Barrios C, Oliva R, Batlle D. Pharmacologic modulation of ACE2 expression. Curr Hypertens Rep 2008;10:410.
- Donoghue M, Hsieh F, Baronas E, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res 2000;87:E1–E9.
- Sama IE, Ravera A, Santema BT, et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin–angiotensin–aldosterone inhibitors. Eur Heart J 2020;41:1810–7.
- Reynolds HR, Adhikari S, Pulgarin C, et al. Renin–angiotensin–aldosterone system inhibitors and risk of Covid-19. N Engl J Med 2020;382:2441-8.
- Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of Covid-19. N Engl J Med 2020;Jun 18 [Epub ahead of print].
- Mehra MR, Desai SS, Kuy SR, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med 2020;382:e102.[Retracted article].
- Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res 2020;126:1671-81.
- Fosbøl EL, Butt JH, Østergaard L, et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA 2020;Jun 19 [Epub ahead of print].
- Cure E, Cumhur Cure M. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may be harmful in patients with diabetes during COVID-19 pandemic. Diabetes Metab Syndr 2020;14:349-50.
- Tadic M, Cuspidi C, Sala C. COVID-19 and diabetes: Is there enough evidence? J Clin Hypertens 2020;22:943–48.
- Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;Mar 25 [Epub ahead of print].
- Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020; Mar 27 [Epub ahead of print].
- Bavishi C, Maddox TM, Messerli FH. Coronavirus disease 2019 (COVID-19) infection and renin angiotensin system blockers. JAMA Cardiol 2020;Apr 3 [Epub ahead of print].
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-62.
- ClinicalTrials.gov. National Institutes of Health, U.S. National Libraries of Medicine. Search of SARS-CoV-2 | Interventional Studies | "COVID-19" "ACE inhibitor." Available at: https://clinicaltrials.gov/ct2/results?term=SARS-CoV-2&cond=%22COVID-19%22+%22ACE+inhibitor%22&age_v=&gndr=&type=Intr&rslt=&Search=Apply. Accessed 06/20/2020.
- ClinicalTrials.gov. National Institutes of Health, U.S. National Libraries of Medicine. Identifier NCT04312009. Losartan for patients with COVID-19 requiring hospitalization. Available at: https://clinicaltrials.gov/ct2/show/NCT04312009. Accessed 06/20/2020.
- ClinicalTrials.gov. National Institutes of Health, U.S. National Libraries of Medicine. Identifier NCT04311177. Losartan for patients with COVID-19 not requiring hospitalization. Available at: https://clinicaltrials.gov/ct2/show/NCT04311177?term=NCT04311177&draw=2&rank=1. Accessed 06/20/2020.
- ClinicalTrials.gov. National Institutes of Health, U.S. National Libraries of Medicine. Identifier NCT04330300. Coronavirus (COVID-19) ACEi/ARB Investigation (CORONACION). Available at: https://clinicaltrials.gov/ct2/show/NCT04330300?term=NCT04330300&draw=2&rank=1. Accessed 06/20/2020.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS Guideline for the prevention, detection, evaluation and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2018;138:e426-e483.
Clinical Topics: COVID-19 Hub, Diabetes and Cardiometabolic Disease, Heart Failure and Cardiomyopathies, Statins
Keywords: Diabetes Mellitus, Metabolic Syndrome, COVID-19, Coronavirus, severe acute respiratory syndrome coronavirus 2, Antihypertensive Agents, Angiotensin Receptor Antagonists, Losartan, Angiotensin-Converting Enzyme Inhibitors, Peptidyl-Dipeptidase A, Chlorthalidone, Angiotensin II, Renin-Angiotensin System, Calcium Channel Blockers, Cardiovascular Diseases, Protein S, Thiazolidines
< Back to Listings