Evolving Therapies in Fontan Failure
What is Fontan Failure?
Fontan failure is a nebulous concept. Heterogeneity of intra-cardiac anatomy, varying Fontan subtypes and overlapping modes of clinical decline make a single, comprehensive definition difficult. In any of its iterations, the Fontan physiology induces maladaptive changes in the heart and in many other organs and bodily systems, with clinical implications that limit health and life expectancy. Fontan failure in childhood has become unusual1 and the burden has shifted into adulthood. The Australian and New Zealand Fontan Registry (ANZFR) defines Fontan failure as the occurrence of death, heart transplant, protein-losing enteropathy (PLE), plastic bronchitis or New York Heart Association (NYHA) Functional class III or IV at follow-up. In 683 adults >16 years, freedom from Fontan failure was 93%, 77%, 62% and 30% at ages 20, 30, 40 and 50 years respectively.2
Management of Atrioventricular Valve Regurgitation
Long recognized as a risk factor for mortality at Fontan completion, atrioventricular valve (AVV) regurgitation is also a critical influence on late outcome. A 2019 ANZFR study of 1,199 Fontan patients who experienced AVV failure had double the risk of subsequent Fontan failure compared to those with competent valves. By age 25, 56% of patients with a common AVV and 46% of those with a single tricuspid valve manifested AVV failure.3 An accompanying editorial discussed important unknowns, especially the potential benefits and optimal timing of AVV repair or replacement.4 Its authors even questioned whether children with important AVV regurgitation, particularly those with a common or tricuspid valve morphology, might better be managed without proceeding to Fontan completion.4 In an era when transcatheter intervention is an accepted therapy for mitral regurgitation in acquired heart disease and is being developed for use on the tricuspid valve,5 non-surgical strategies to improve AVV regurgitation may be attempted in carefully selected Fontan patients in the future.
Pulmonary Vasodilator Therapy
The relationship between pulmonary vascular disease and Fontan failure is complex and overlapping. Non-pulsatile pulmonary blood flow (PBF), thrombosis, AVV regurgitation and diastolic ventricular dysfunction all affect pulmonary vasculature. Even small increases in pulmonary vascular resistance reduce PBF, limit ventricular preload and cardiac output, and elevate central venous pressures. In a 2017 study of 261 adult Fontan patients, those with elevated pulmonary vascular resistance (>2 WU.m2) and low cardiac index (<2.5L/min/m2) were at increased risk of Fontan failure (death, transplant listing or initiation of palliative care) within five years.6 A 2019 meta-analysis of nine, small randomized controlled trials of various pulmonary vasodilators in 381 Fontan patients determined that pulmonary vasodilators led to improvement in peak V02, NYHA class, six-minute walking distance performance and hemodynamics, without affecting mortality.7 Although the jury is out, it seems that perhaps the theoretical advantages and "signal of benefit" for pulmonary vasodilators in Fontan failure may be stronger than those for other medical heart failure therapies.
External Ventilation
In a Fontan circulation, systemic venous and PBF patterns are related to breathing, and respiration affects the ability to exercise.8 Inspiration increases systemic venous flow amplitudes and venous pulsatility, although net flow seems only minimally influenced.9,10 The adverse impact of positive pressure ventilation on Fontan hemodynamics has long been recognized, and negative pressure ventilation strategies increase PBF and cardiac output after Fontan completion surgery.11,12 Lately, external ventilation devices that might be worn outside of a hospital setting have sparked some interest. Our group recently conducted a prospective pilot study of external negative and biphasic pressure in 10 healthy adult Fontan patients and 10 controls.13 We found that PBF and cardiac output increased during both modes of external ventilation, with a greater change and better tolerance seen with the biphasic setting.13 It is tempting to speculate that external ventilation might help failing Fontan physiology, though caution is required, and further study is essential. Respiratory driven retrograde hepatic flow is substantial in Fontan patients.10 Further augmentation by deepening inspiration might potentially increase hepatic afterload and theoretically worsen Fontan associated liver disease.10
The New Focus on Lymphatics
Blood is not the only substance that circulates. The past decade has revealed that the lymphatic system is intrinsically abnormal or specifically maladapted in many Fontan patients. Increased venous pressures not only enhance lymphatic production, but also obstruct emptying of the thoracic duct,14 mechanisms which may contribute to complications such as PLE, chylothorax, plastic bronchitis and ascites. Of tiny caliber and without a pumping ventricle, it may be a surprise that lymphatic vessels are highly active, with contractile walls propelling lymph back towards the central veins.15,16 Recent innovative studies from a Danish group demonstrated structural lymphatic changes and diminished lymphangion pumping in apparently healthy Fontan patients.17 Therapeutic options targeting lymphatics have been successful in small numbers of children and include surgical decompression of the thoracic duct18 and occlusive embolization of dilated lymphatic channels.19 Further characterization of lymphatic abnormalities may lead to preventative measures and new management options for what are currently the most challenging Fontan complications.
Transplant and Mechanical Support
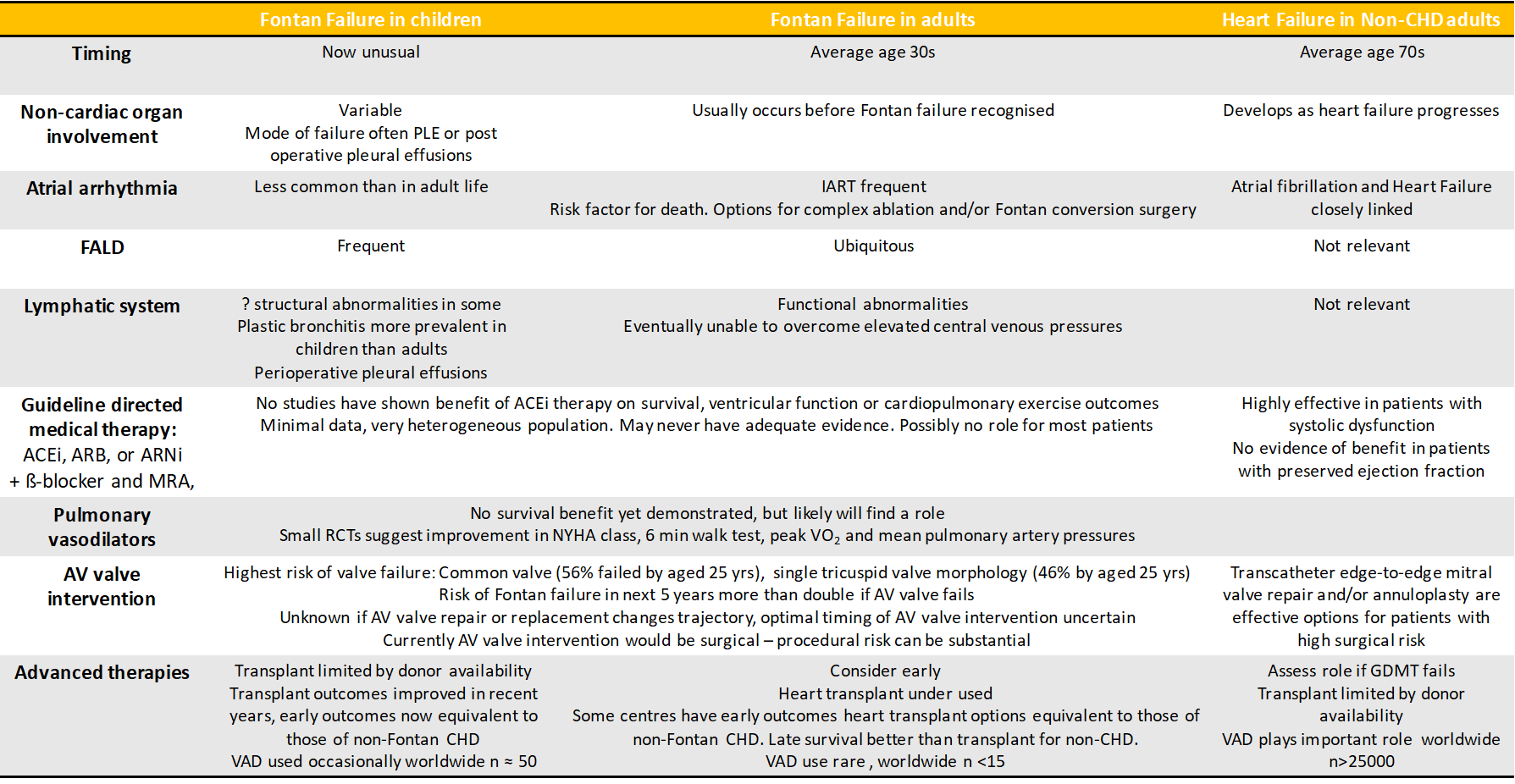
Heart transplant for the failing Fontan circulation is effective therapy, but is challenging and in the current era, may be underutilized.20,21 Previously, survival after heart transplantation in Fontan patients was worse than that of other non-Fontan congenital heart disease recipients.22,23 More recent data shows an improvement and some groups now achieve Fontan heart transplant outcomes at par with those for other forms of congenital heart disease.24-26 Optimization of Fontan transplant outcomes requires concerted, multidisciplinary effort and the development of specific expertise,21 but is a worthy endeavor given the potential for excellent long-term survival.27 Some groups have demonstrated low mortality by opting for combined heart-liver transplant in the majority of Fontan recipients.28 The merits and disadvantages of such an approach warrant further evaluation. Experience suggests that in Fontan patients, even advanced liver fibrosis can regress after heart transplant,29 and there is data supporting equivalent 1-year post heart transplant outcomes for cirrhotic and non-cirrhotic Fontan patients.30 Heart transplant is also an effective treatment for PLE and in children, the severity and duration of PLE are not associated with worse post-transplant outcomes.31 Durable mechanical support options for Fontan circulations exist and are under further development. However, due to current technical and other challenges, their use is not widespread.32 Most Fontan failure now occurs in adult life.1 Traditional training pathways for advanced heart failure care, pediatric cardiology and/or adult congenital heart disease are quite different, and none is ideally suited to encompass all aspects of care these patients need. The failing Fontan patient is unique (Figure 1). A team approach, involving pediatric and adult congenital heart disease and advanced heart failure services, is essential (Figure 1).
Figure 1: Similarities and differences between Fontan Failure in children and adults and Heart Failure in adults without congenital heart disease
CHD – congenital heart disease, PLE - protein losing enteropathy, IART – interatrial reentry tachycardia, ACEi – angiotensin converting enzyme inhibitor, ARB – angiotensin receptor blocker, ANRi – angiotensin receptor-neprilysin inhibitor, b-blocker – beta-adrenergic blocking agent, MRA – mineralocorticoid receptor antagonist, NYHA – New York Heart Association, Peak VO2 – peak oxygen uptake, VAD – ventricular assist device
Evolution of care
In the current era, the transition of pediatric Fontan patients to effective adult congenital heart disease care, which maintains lifelong access to multidisciplinary expertise, specialist imaging, and advanced and emerging management options, is essential.33 Conversely, our growing experience with adult Fontan survivors should prompt ongoing critical evaluation of pediatric practices and inform our medical and surgical strategies for the next generation.
References
- Kotani Y, Chetan D, Zhu J, et al. Fontan failure and death in contemporary Fontan circulation: analysis from the last two decades. Ann Thorac Surg 2018;105:1240–7.
- Dennis M, Zannino D, Plessis du K, et al. Clinical outcomes in adolescents and adults after the Fontan procedure. J Am Coll Cardiol 2018;71:1009–17.
- King G, Ayer J, Celermajer D, et al. Atrioventricular valve failure in Fontan palliation. J Am Coll Cardiol 2019;73:810–22.
- Cetta F, Driscoll DJ. Bad atrioventricular valve, bad Fontan: stop creating bad fontans. J Am Coll Cardiol 2019;73:823–5.
- Tabata N, Sugiura A, Tsujita K, Nickenig G, Sinning J-M. Percutaneous interventions for mitral and tricuspid heart valve diseases. Cardiovasc Interv Ther 2020;35:62–71.
- Egbe AC, Connolly HM, Miranda WR, et al. Hemodynamics of Fontan failure: the role of pulmonary vascular disease Circ Heart Fail 2017;10:503–8.
- Wang W, Hu X, Liao W, et al. The efficacy and safety of pulmonary vasodilators in patients with Fontan circulation: a meta-analysis of randomized controlled trials. Pulm Circ 2019;9:2045894018790450.
- Van De Bruaene A, Kutty S. The peculiar challenges of breathing and exercising with a Fontan circulation. Am J Physiol Heart Circ Physiol 2019;316:H311–H313.
- Wei Z, Whitehead KK, Khiabani RH, et al. Respiratory effects on Fontan circulation during rest and exercise using real-time cardiac magnetic resonance imaging. Ann Thorac Surg 2016;101:1818–25.
- Gabbert DD, Hart C, Jerosch-Herold M, et al. Heartbeat but not respiration is the main driving force of the systemic venous return in the Fontan circulation. Scientific Reports 2019;9:2034.
- Shekerdemian LS, Bush A, Shore DF, Lincoln C, Redington AN. Cardiopulmonary interactions after Fontan operations: augmentation of cardiac output using negative pressure ventilation. Circulation 1997;96:3934–42.
- Shekerdemian LS, Bush A, Shore DF, Lincoln C, Redington A. Cardiorespiratory responses to negative pressure ventilation after tetralogy of fallot repair: a hemodynamic tool for patients with a low-output state. J Am Coll Cardiol 1999;33:549–55.
- Charla P, Yamamura K, Kaur GR, et al. Abstract 143275: Feasibility and efficacy of negative pressure ventilation in the ambulatory Fontan population-(FONTAN-CMR)-a pilot study. Circulation 2018;138:A14275.
- Menon S, Chennapragada M, Ugaki S, Sholler GF, Ayer J, Winlaw DS. The lymphatic circulation in adaptations to the Fontan circulation. Pediatr Cardiol 2017;38:886–92.
- Telinius N, Baandrup U, Rumessen J, et al. The human thoracic duct is functionally innervated by adrenergic nerves. AJP Heart and Circulatory Physiology 2014;306:H206–13.
- Telinius N, Majgaard J, Kim S, et al. Voltage-gated sodium channels contribute to action potentials and spontaneous contractility in isolated human lymphatic vessels. J Physiol 2015;593:3109–22.
- Mohanakumar S, Telinius N, Kelly B, et al. Morphology and function of the lymphatic vasculature in patients with a Fontan circulation. Circ Cardiovasc Imaging 2019;12:e008074.
- Hraska V. Decompression of thoracic duct: new approach for the treatment of failing Fontan. Ann Thorac Surg 2013;96:709–11.
- Dori Y, Keller MS, Rome JJ, et al. Percutaneous lymphatic embolization of abnormal pulmonary lymphatic flow as treatment of plastic bronchitis in patients with congenital heart disease. Circulation 2016;133:1160–70.
- Kenny LA, DeRita F, Nassar M, Dark J, Coats L, Hasan A. Transplantation in the single ventricle population. Ann Cardiothorac Surg 2018;7:152–9.
- Crossland DS, Van De Bruaene A, Silversides CK, Hickey EJ, Roche SL. Heart failure in adult congenital heart disease: from advanced therapies to end-of-life care. Can J Cardiol 2019;35:1723–39.
- Doumouras BS, Alba AC, Foroutan F, Burchill LJ, Dipchand AI, Ross HJ. Outcomes in adult congenital heart disease patients undergoing heart transplantation: a systematic review and meta-analysis. J Heart Lung Transplant 2016;35:1337–47.
- Bernstein D, Naftel D, Chin C, et al. Outcome of listing for cardiac transplantation for failed fontan: a multi-institutional study. Circulation 2006;114:273–80.
- Simpson KE, Pruitt E, Kirklin JK, et al. Fontan patient survival after pediatric heart transplantation has improved in the current era. Ann Thorac Surg 2017;103:1315-20.
- Crossland DS, Jansen K, Parry G, et al. Outcome following heart transplant assessment in adults with congenital heart disease. Heart 2019;105:1741–7.
- Tabarsi N, Guan M, Simmonds J, et al. Meta-analysis of the effectiveness of heart transplantation in patients with a failing fontan. Am J Cardiol 2017;119:1269–74.
- Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult heart transplantation report - 2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1056–66.
- Reardon LC, DePasquale EC, Tarabay J, et al. Heart and heart-liver transplantation in adults with failing Fontan physiology. Clin Transplant 2018;32:e13329.
- Bouchardy J, Meyer P, Yerly P, et al. Regression of advanced liver fibrosis after heart transplantation in a patient with prior Fontan surgery for complex congenital heart disease. Circ Heart Fail 2018;11:1–3.
- Simpson KE, Esmaeeli A, Khanna G, et al. Liver cirrhosis in Fontan patients does not affect 1-year post-heart transplant mortality or markers of liver function. J Heart Lung Transplant 2014;33:170–7.
- Schumacher KR, Yu S, Butts R, et al. Fontan-associated protein-losing enteropathy and post‒heart transplant outcomes: a multicenter study. J Heart Lung Transplant 2019;38:17–25.
- Buratto E, Shi WY, Ye XT, Konstantinov IE. Ventricular assist devices for the failing univentricular circulation. Expert Review of Medical Devices 2017;14:449–59.
- Rychik J, Atz AM, Celermajer DS, et al. Evaluation and management of the child and adult with Fontan circulation: a scientific statement from the American Heart Association. Circulation 2019;140:613–51.
Clinical Topics: Cardiac Surgery, Congenital Heart Disease and Pediatric Cardiology, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Valvular Heart Disease, Cardiac Surgery and CHD and Pediatrics, Cardiac Surgery and Heart Failure, Cardiac Surgery and VHD, Congenital Heart Disease, CHD and Pediatrics and Interventions, CHD and Pediatrics and Prevention, CHD and Pediatrics and Quality Improvement, Interventions and Structural Heart Disease, Mitral Regurgitation
Keywords: Heart Defects, Congenital, Fontan Procedure, Protein-Losing Enteropathies, Thoracic Duct, Central Venous Pressure, Liver Transplantation, Tricuspid Valve, Risk Factors, Chylothorax, Ascites, Mitral Valve Insufficiency, Prospective Studies, Vasodilator Agents, Palliative Care, Life Expectancy, Pilot Projects
< Back to Listings