Intravascular Lithotripsy in Cardiovascular Interventions
Introduction
Moderate to severe calcification, which is present in one-third of patients presenting with stable disease or acute coronary syndromes1 and in up to half of revascularization procedures in peripheral arteries,2,3 portends worse procedural success and an increase in periprocedural rates of major adverse events and long-term rates of in-stent restenosis, stent thrombosis, and target and lesion revascularization.1,4 A promising new addition to the armamentarium for treatment of severely calcified lesions in the coronary and peripheral vasculature is the adaptation of lithotripsy technology for vascular calcification. Lithoplasty was the first term used for application of lithotripsy in angioplasty and has been replaced by the term intravascular lithotripsy (IVL).
Calcium Modification by Shockwave Lithotripsy
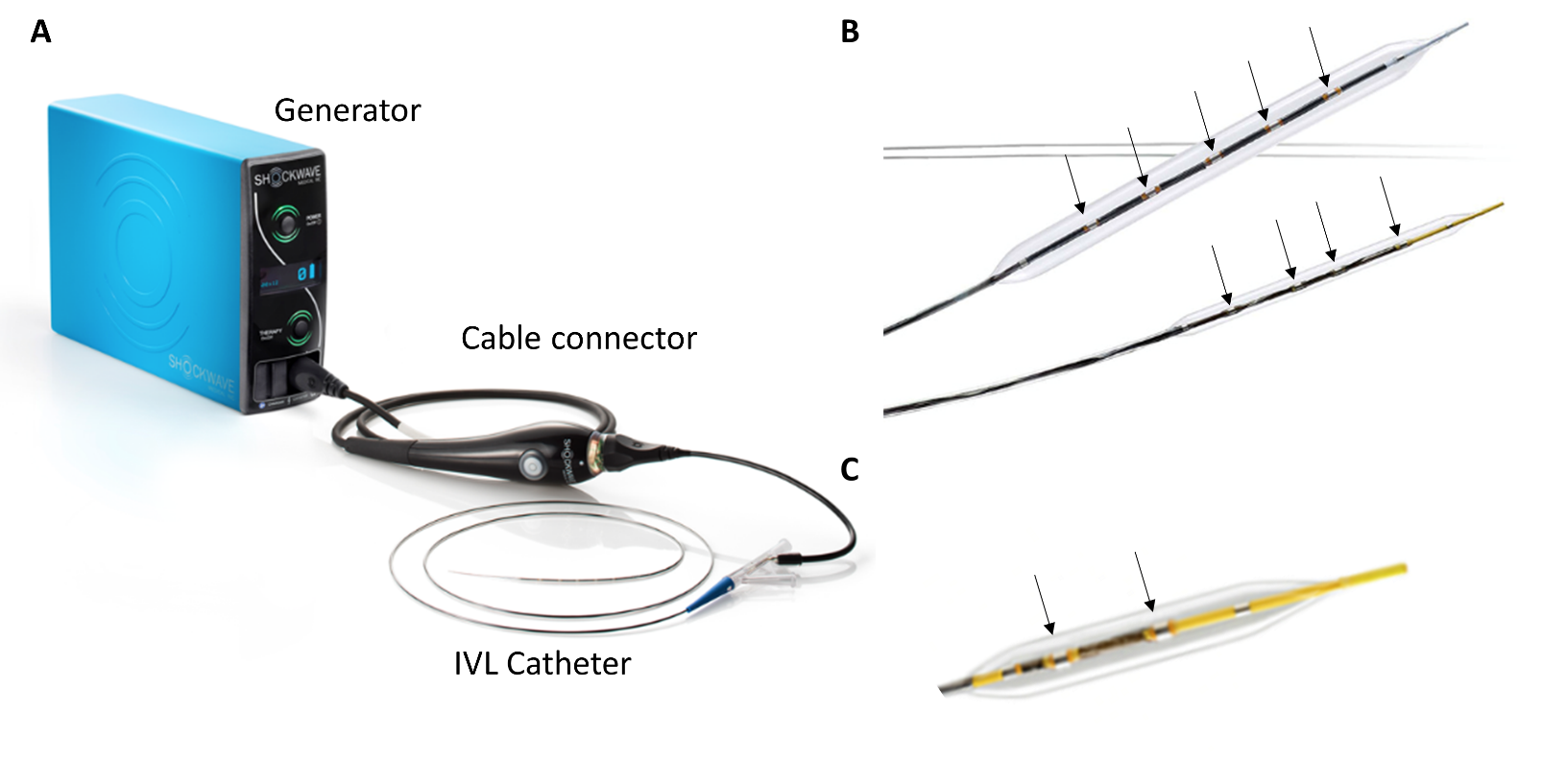
The IVL balloon-catheter system includes miniaturized and arrayed lithotripters that are integrated into a semi-compliant balloon filled with a mixture of contrast and saline (Figure 1). The lithotripters generate shock waves that are similar in their waveform to the shock waves generated by the lithotripters used in the extracorporeal lithotripsy of nephrolithiasis. These shock waves are characterized by short duration of ~5 mcs, with positive pressure peak and negative pressure trough components.5 The IVL shock waves generate a peak positive pressure of ≈50 atm.6 The contrast-filled balloon is inflated at a subnominal pressure (4 atm) and apposed to the vessel wall, thus providing an effective fluid-tissue interface with similar acoustic impedances that facilitates efficient coupling of the shock wave energy to the vessel wall.
Figure 1: IVL Set-Up for Peripheral and Coronary Intervention
Several mechanisms may play a role in calcific plaque fragmentation by IVL, including axial splitting by compressive circumferential forces, generation and violent collapse of cavitation bubbles by shock waves inside the saline-contrast filled balloon that impact the surface of the calcific plaque, and fatigue mechanism involving progressive expansion of microfractures into macrofractures by the cumulative impact of the repetitive multiple shock wave pulses.
IVL in Coronary Artery Disease
IVL use in the coronary circulation has been tested in the European pre-marketing Disrupt CAD I (Shockwave Coronary Rx Lithoplasty® Study)7 leading to CE mark approval for treatment of severely calcified coronary lesions. The coronary IVL set-up, Shockwave Coronary Rx Lithoplasty System (Shockwave Medical; Santa Clara, CA), consists of a portable and battery-chargeable generator, a cable connector with a push button for IVL activation, and the IVL balloon-catheter (Figure 1). The coronary IVL system has two emitters integrated on a rapid exchange balloon-based system and is available in diameters from 2.5 mm to 4.0 mm (in 0.5-mm increments) and is 12 mm in length. The IVL balloon is selected in a 1:1 ratio to the reference coronary diameter, often guided by intravascular imaging, which is recommended for optimal lesion preparation. The coronary IVL generator is preprogrammed to deliver 10 pulses in sequence at a frequency of 1 pulse/second for a maximum of 80 pulses per catheter.
The results of trials assessing the safety of efficacy of IVL in coronary circulation are summarized in Table 1. Disrupt CAD I was a single-arm, non-randomized study that enrolled 60 patients with severely calcified coronary lesions.7 IVL was feasible in all patients and facilitated delivery of stents to all target lesions. The average stenosis was reduced to 12% with an acute diameter gain of 1.7 mm, thus achieving 95% clinical success (defined as residual diameter stenosis <50% without in-hospital major adverse cardiovascular events [MACE]). The procedure was safe, with no unresolved dissections, slow flow/no reflow, embolization, or perforations.7
Table 1: Coronary IVL Studies
| Disrupt CAD I | Disrupt CAD II | |
| Design | Multicenter, single arm | Multicenter, single arm |
| Number of Patients | 60 | 120 |
| Number of Sites | 7 | 15 |
| Inclusion Criteria |
|
|
| Acute Gain | 1.7 mm | 1.6 mm |
| 30-Day MACE | 5.0% | 7.6% |
| 6-Month MACE | 8.3% | — |
In the optical coherence tomography substudy of Disrupt CAD I, calcium fracture was seen along the circumference of the calcified plaques, and multiple fractures in a single cross-section were detected in >25% of lesions, leading to an average acute area gain of ~2.1 mm2 with IVL alone.8 IVL-induced fractures were independent of calcium depth, with multiple fractures per lesion occurring more frequently as the severity of the underlying calcification increased. IVL-facilitated percutaneous coronary intervention resulted in stent apposition and expansion approaching those observed in contemporary series of drug-eluting stent implantation in less complex and non-calcified lesions.
In Disrupt CAD II, the IVL catheter was successfully delivered to all target lesions. The post-IVL angiographic acute luminal gain was 0.83 ± 0.47 mm, and residual stenosis was 32.7 ± 10.4%, which further decreased to 7.8 ± 7.1% after drug-eluting stent implantation. The primary safety endpoint occurred in 5.8% of patients, consisting of 7 non-Q-wave myocardial infarctions. There were no incidences of procedural abrupt closure, slow flow/no reflow, or perforation. Disrupt CAD III (NCT03595176; n = 384 patients), which recently completed enrollment, and Disrupt CAD IV (NCT04151628; n = 64 patients) are designed for US Food and Drug Administration (FDA) approval and pre-marketing approval in Japan, respectively.
Clinical experience with IVL has shown that IVL may induce calcium fracture where rotational atherectomy has failed to adequately modify the calcium.9 IVL has also been used in in-stent restenosis due to calcified neoatherosclerosis10 or underexpanded stents implanted in severely calcified lesions,11-13 where the metallic scaffold impedes interaction of devices such as rotational atherectomy, cutting or scoring balloons with the calcified plaque.
Effects of Shock Waves on Cardiac Rhythm
Acoustic shockwaves of IVL can induce localized myocardial depolarization, likely by activation of mechano-sensitive cardiomyocyte membrane ion channels, resulting in atrial or ventricular ectopic beats ("shocktopics"), either as isolated capture beats or asynchronous cardiac pacing (≥2 beats).14 Although there is a theoretical risk of inducing tachyarrhythmias if the capture occurs during the vulnerable phase of repolarization, no ventricular tachyarrhythmias induced by IVL have so far been reported. Disrupt CAD III will systematically report the effects of IVL on cardiac rhythm.
IVL in Peripheral Arterial Disease
The Shockwave Peripheral IVL System (Shockwave Medical; Santa Clara, CA) is similar to the coronary IVL system but has 4 or 5 lithotripters (Figure 1). The initial FDA-cleared Shockwave Peripheral IVL System consisted of an over-the-wire balloon ranging from 3.5 to 7.0 mm diameter (0.5-mm increments) and 60 mm in length (referred to as M5), requiring a 6F or 7F sheath depending on the balloon diameter. Recently, 4 additional catheter sizes (2.5 mm, 3.0 mm, 3.5 mm, and 4.0 mm in diameter, 40 mm in length and 5F sheath compatible, referred to as S4) have been approved. The smaller IVL balloon-catheter (S4) has a lower crossing profile, hydrophilic coating, and a longer shaft length (135 cm) with increased flexibility and pushability compared with the M5 catheter, providing a superior ability to go "up and over" across the aorto-iliac bifurcation to treat contralateral distal peripheral calcified lesions. The S4 catheter also has different lithotripter spacing compared with the first-generation M5 catheter, with the middle 2 of the 4 lithotripters of the S4 catheter placed closer to each other to optimize the impact IVL on the target calcified plaques (Figure 1).
To deliver the shock waves, the saline-contrast filled balloon is inflated at a subnominal pressure (4 atm) in the target vessel, and the shock wave pulses are generated by pressing and holding the push button to activate the IVL generator. The IVL generator is pre-programmed to deliver shock wave pulses at specified time intervals (20 or 30 seconds) and number of pulses (160 or 300) per catheter. The IVL cycles are repeated until the target lesion is deemed adequately modified.
The peripheral IVL catheter is approved in Europe and in the United States for elective treatment of various peripheral vascular beds including the iliac, femoral, popliteal, infra-popliteal, mesenteric, celiac, and renal arteries. The results of the clinical trials examining the safety and efficacy of IVL in various peripheral vascular beds, namely Disrupt PAD I (Safety and Performance Study of the Shockwave Lithoplasty System), Disrupt PAD II (Shockwave Lithoplasty DISRUPT Trial for PAD), Disrupt PAD III (Shockwave Medical Peripheral Lithoplasty System Study for PAD), and Disrupt BTK (Safety and Feasibility of the Shockwave Lithoplasty® System for the Treatment of Peripheral Vascular Stenosis), are summarized in Table 2. A meta-analysis of the pooled individual patient-level data from 5 studies of IVL in various peripheral vascular territories (including 336 patients) noted a significant reduction of 55.1% in diameter stenosis by IVL (95% confidence interval, 53.3-57.0%; p < 0.0001) and an overall mean final diameter stenosis of 23.7%.15 Core laboratory-assessed lesion-level complications occurred in 4/328 (1.22%) of the treated lesions, which included flow-limiting dissections (types D-F; n = 3), vessel perforation (n = 1, due to drug-coated balloon inflation and not IVL), with no distal embolization, thrombus, abrupt closure, or no-reflow events.
Table 2: Peripheral IVL Studies
| Disrupt PAD I/II | Disrupt PAD III | Disrupt PAD III Observational Registry | Disrupt BTK | |
| Design | Multicenter, single-arm | Multicenter, single-arm | Multicenter, single-arm | Multicenter, single-arm |
| Number of Patients | PAD I: 35 PAD II: 60 |
200 | 118 | 20 |
| Number of Sites | PAD I: 3 PAD II: 8 |
18 | 20 | 3 |
| Inclusion Criteria |
|
|
|
|
| Acute Gain | PAD I: 2.9 mm PAD II: 3.0 mm |
3.4 mm | — | 1.5 mm |
| 30-Day Major Adverse Events | PAD I: 0.0% PAD II: 1.7% |
— | — | 0.0% |
| 6-Month Major Adverse Events | PAD I: 0.0% PAD II: 1.7% |
— | — | — |
IVL-Facilitated Vascular Access
IVL has proven useful in treating calcific iliofemoral disease to facilitate the passage of the large-bore delivery sheaths for transcatheter aortic valve replacement.16-21 IVL use for this purpose obviates the need for alternative access sites that can result in greater adverse outcomes. Similarly, reports of IVL-facilitated transfemoral access for endovascular aneurysm repair16,20,21 or for placement of mechanical circulatory support devices22 have demonstrated that IVL can be a valuable addition in these procedures for management of patients with heavily calcified "prohibitive" transfemoral access.
Conclusions
IVL is an intuitive and attractive modality for the treatment of severely calcified lesion that combines the calcium-disrupting capability of lithotripsy with the familiarity of balloon catheters. Clinical studies to date support the effectiveness of IVL in inducing circumferential fracture in calcific plaques, leading to significant luminal gain and facilitating optimal stent expansion. Ongoing clinical studies will add further insight into the safety and effectiveness of the technique and determine the place for IVL in the algorithms for treatment of heavily calcified lesions in coronary and peripheral circulation.
References
- Généreux P, Madhavan MV, Mintz GS, et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) TRIALS. J Am Coll Cardiol 2014;63:1845-54.
- Walker KL, Nolan BW, Columbo JA, et al. Lesion complexity drives the cost of superficial femoral artery endovascular interventions. J Vasc Surg 2015;62:998-1002.
- Soor GS, Vukin I, Leong SW, Oreopoulos G, Butany J. Peripheral vascular disease: who gets it and why? A histomorphological analysis of 261 arterial segments from 58 cases. Pathology 2008;40:385-91.
- Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary Artery Calcification and its Progression: What Does it Really Mean? JACC Cardiovasc Imaging 2018;11:127-42.
- Cleveland RO, McAteer JA. Physics of Shock-Wave Lithotripsy. In: Smith AD, eds. Smith's Textbook of Endourology, 3rd Edition. Hoboken: Wiley-Blackwell; 2011:529-558.
- Dini CS, Tomberli B, Mattesini A, et al. Intravascular lithotripsy for calcific coronary and peripheral artery stenoses. EuroIntervention 2019;15:714-21.
- Brinton TJ, Ali ZA, Hill JM, et al. Feasibility of Shockwave Coronary Intravascular Lithotripsy for the Treatment of Calcified Coronary Stenoses. Circulation 2019;139:834-6.
- Ali ZA, Brinton TJ, Hill JM, et al. Optical Coherence Tomography Characterization of Coronary Lithoplasty for Treatment of Calcified Lesions: First Description. JACC Cardiovascular Imaging 2017;10:897-906.
- Ielasi A, Loffi M, De Blasio G, Tespili M. "Rota-tripsy": A successful combined approach for the treatment of a long and heavily calcified coronary lesion. Cardiovasc Revasc Med 2019;Dec 18:[Epub ahead of print].
- Salazar C, Escaned J, Tirado G, Gonzalo N. Recurrent restenosis caused by severe calcific neoatherosclerosis treated with intravascular lithotripsy. EuroIntervention 2019;Jul 23:[Epub ahead of print].
- Ali ZA, McEntegart M, Hill JM, Spratt JC. Intravascular lithotripsy for treatment of stent underexpansion secondary to severe coronary calcification. Eur Heart J 2020;41:485-6.
- Tovar Forero MN, Wilschut J, Van Mieghem NM, Daemen J. Coronary lithoplasty: a novel treatment for stent underexpansion. Eur Heart J 2019;40:221.
- Alfonso F, Bastante T, Antuña P, et al. Coronary Lithoplasty for the Treatment of Undilatable Calcified De Novo and In-Stent Restenosis Lesions. JACC Cardiovasc Interv 2019;12:497-9.
- Wilson SJ, Spratt JC, Hill J, et al. Incidence of "shocktopics" and asynchronous cardiac pacing in patients undergoing coronary intravascular lithotripsy. EuroIntervention 2020;15:1429-35.
- Madhavan MV, Shahim B, Mena-Hurtado C, Garcia L, Crowley A, Parikh SA. Efficacy and safety of intravascular lithotripsy for the treatment of peripheral arterial disease: An individual patient-level pooled data analysis. Catheter Cardiovasc Interv 2020;95:959-68.
- Armstrong EJ, Soukas PA, Shammas N, et al. Intravascular lithotripsy for treatment of calcified, stenotic iliac arteries: A cohort analysis from the Disrupt PAD III Study. Cardiovasc Revasc Med 2020;Mar 2:[Epub ahead of print].
- Di Mario C, Goodwin M, Ristalli F, et al. A Prospective Registry of Intravascular Lithotripsy-Enabled Vascular Access for Transfemoral Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2019;12:502-4.
- Gorla R, Cannone GS, Bedogni F, De Marco F. Transfemoral aortic valve implantation following lithoplasty of iliac artery in a patient with poor vascular access. Catheter Cardiovasc Interv 2019;93:E140-E142.
- Cruz-González I, González Ferreiro R, Moreiras JM, et al. Facilitated Transfemoral Access by Shockwave Lithoplasty for Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2019;12:e35-e38.
- Khalid N, Iantorno M, Shlofmitz E, Hashim H, Waksman R, Bernardo N. Kissing Intravascular Lithotripsy Facilitated Endovascular Repair of a Complex Saccular Abdominal Aortic Aneurysm With Narrowed Distal Aorta: A First-in-Human Report. JACC Cardiovasc Interv 2019;12:e97-e99.
- Rosseel L, De Backer O, Søndergaard L, Bieliauskas G. Intravascular iliac artery lithotripsy to enable transfemoral thoracic endovascular aortic repair. Catheter Cardiovasc Interv 2020;95:E96-E99.
- Riley RF, Corl JD, Kereiakes DJ. Intravascular lithotripsy-assisted Impella insertion: A case report. Catheter Cardiovasc Interv 2019;93:1317-9.
Clinical Topics: Arrhythmias and Clinical EP, Cardiac Surgery, Dyslipidemia, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Stable Ischemic Heart Disease, Atherosclerotic Disease (CAD/PAD), EP Basic Science, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Aortic Surgery, Cardiac Surgery and Arrhythmias, Cardiac Surgery and SIHD, Lipid Metabolism, Interventions and Coronary Artery Disease, Interventions and Imaging, Interventions and Vascular Medicine, Angiography, Nuclear Imaging, Chronic Angina
Keywords: Coronary Angiography, Peripheral Arterial Disease, Ventricular Premature Complexes, Renal Artery, Tachycardia, Myocytes, Cardiac, Atrial Fibrillation, Myocardium, Ion Channels, Atherectomy, Coronary, Coronary Artery Disease, Drug-Eluting Stents, Constriction, Pathologic, Tomography, Optical Coherence, United States Food and Drug Administration, Electric Impedance, Fractures, Stress, Vascular Calcification, Coronary Restenosis, Percutaneous Coronary Intervention, Lithotripsy, Calcium
< Back to Listings