The NCDR Left Atrial Appendage Occlusion Registry
Introduction
Atrial fibrillation (AF) confers a four- to fivefold increased risk for ischemic stroke and accounts for approximately 15% of ischemic strokes in the United States each year. Long-term anticoagulation with warfarin or direct oral anticoagulants is the standard of care for stroke prevention for individuals with nonvalvular AF and a moderate or high stroke risk. Left atrial appendage occlusion (LAAO) lowers the risk of stroke by excluding the left atrial appendage from the systemic circulation and preventing thrombus formation and embolization; it has emerged as a treatment option for patients with AF who are at moderate to high risk of stroke and poor candidates for long-term anticoagulation.
Prior Clinical Trials and NCDR LAAO Registry
The PROTECT-AF (WATCHMAN Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation; n = 707) and PREVAIL (Prospective Randomized Evaluation of the WATCHMAN LAA Closure Device in Patients With Atrial Fibrillation; n = 407) pivotal trials were relatively small randomized controlled trials designed as Bayesian non-inferiority trials comparing the WATCHMAN device to warfarin anticoagulation in patients eligible for both over the long term. In March 2015, after these trials with accompanying continued access protocol data, the WATCHMAN™ LAAO device (Boston Scientific; Marlborough, MA) was approved by the US Food and Drug Administration (FDA) for stroke prevention in AF. Several other percutaneous LAAO devices are currently being developed and evaluated in clinical trials.
To better understand the utilization, safety and effectiveness of LAAO devices in real-world clinical practice, the American College of Cardiology and the Society for Coronary Angiography and Intervention collaborated with the FDA, the Centers for Medicare and Medicaid Services, and Boston Scientific to develop the National Cardiovascular Data Registry (NCDR) Left Atrial Appendage Occlusion Registry (LAAO Registry). Here we present the patient, hospital, and physician characteristics as well as in-hospital adverse event rates for WATCHMAN percutaneous LAAO procedures performed in the United States during the first 3 years of the LAAO Registry between 2016 and 2018.
Key Findings
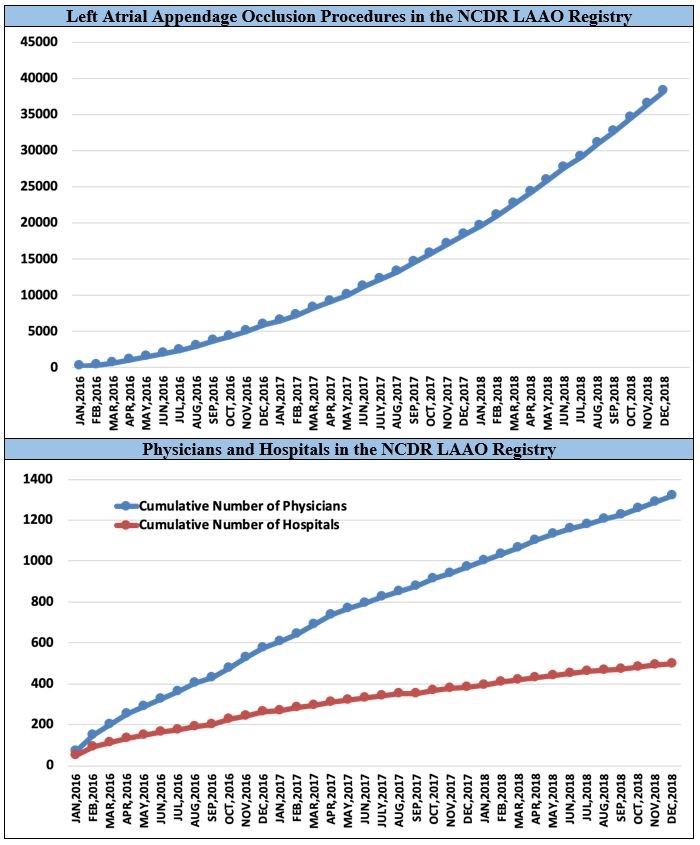
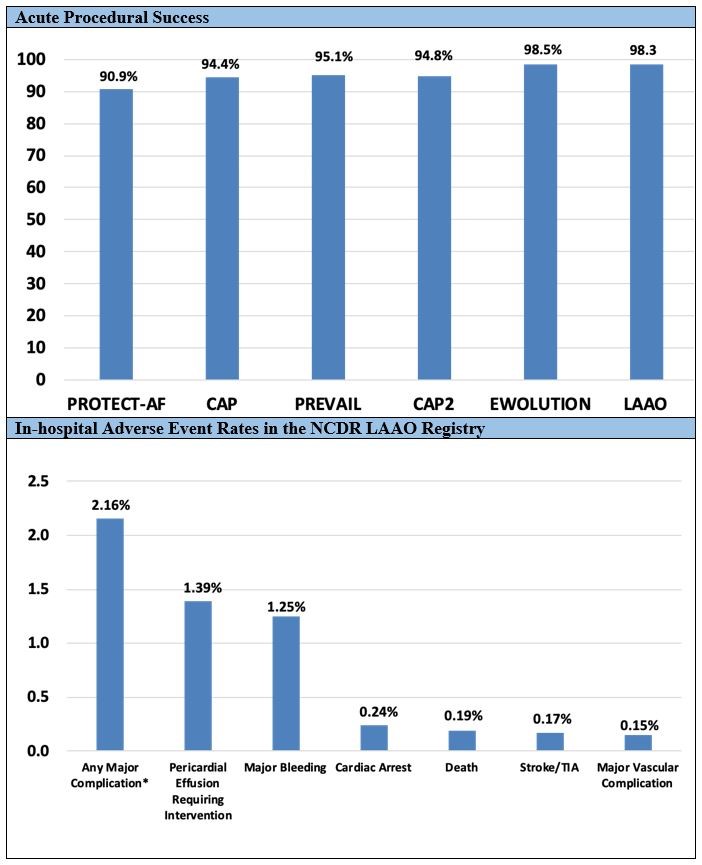
A total of 38,158 procedures from 495 hospitals performed by 1,318 physicians in the United States was enrolled in the NCDR LAAO Registry between January 2016 and December 2018. The mean patient age was 76.1 ± 8.1 years, the mean CHA2DS2-VASc score was 4.6 ± 1.5, and the mean HAS-BLED score was 3.0 ± 1.1. The median annual number of LAAO procedures performed for hospitals was 30 (interquartile range = 18-44), with most hospitals performing fewer than 40 procedures per year. For physicians, the median annual volume was 12 (interquartile range = 8-20), with most performing fewer than 20 procedures per year. However, there was substantial variation in volumes across sites and providers (Figure1). Procedures were cancelled or aborted in 7% of cases; among cases in which a device was deployed, 98.1% were implanted with <5 mm leak. Major in-hospital adverse events occurred in 2.16% of patients. The most common complications were pericardial effusion requiring intervention (1.39%) and major bleeding (1.25%); stroke (0.17%) and death (0.19%) were rare (Figure 2).
Figure 1: Procedure Volume, Implanting Physicians, and Implanting Hospitals in the NCDR LAAO Registry, 2016-2018
Figure 2: Acute Procedural Success Compared With Prior Trials and Registries and Major In-Hospital Adverse Events in the NCDR LAAO Registry, 2016-2018
Discussion
These data from the LAAO Registry demonstrate that patients undergoing commercial WATCHMAN left atrial appendage closure in the United States are older and at higher thromboembolic and bleeding risk than individuals participating in the pivotal trials and most earlier registries, with a mean CHA2DS2-VASc score of 4.6 and a mean HAS-BLED score of 3. Most patients in the LAAO Registry had relative or absolute contraindications to long-term anticoagulation, including a 69% rate of prior bleeding and a 12% rate of intracranial bleeding. In comparison, only 13.3% of the patients enrolled in the PROTECT-AF and PREVAIL randomized clinical trials had a prior bleeding event.
In the PROTECT-AF trial, a device was successfully implanted in 88% (408/463) of patients assigned to LAAO intervention and in 90.9% (408/449) of those in whom implantation was attempted (Figure 2). In the PREVAIL trial, implantation was performed in 95.1% of those in whom it was attempted, suggesting improvement in procedural technique and operator experience overall. In EWOLUTION (Registry on WATCHMAN Outcomes in Real-Life Utilization), a device was successfully deployed in 98.5% of patients, and 0.7% had a residual leak >5 mm, which is comparable to our findings. Our study showed that implantation success rates in contemporary practice are higher than in the PROTECT-AF trial, the PREVAIL trial, and the Continued Access Protocol registries and comparable to the more recent EWOLUTION registry, reflecting possible improvement in patient selection, procedural protocols, and operator technique over time.
The pivotal WATCHMAN trials reported 7-day procedure-related adverse events, and the LAAO Registry collects adverse events during the index hospitalization; therefore, some procedure-related adverse events may not be captured until the 45 days follow-up time point. However, most major procedure-related adverse events occur acutely and will be detected during the index hospitalization, and event rates are broadly comparable to the 7-day event rates reported in the trials. We found that rates of in-hospital major adverse events were substantially lower than the 7-day procedure-related adverse events reported in the PROTECT-AF trial (pericardial effusion requiring surgery or pericardiocentesis = 4%; major bleeding = 3.5%; procedure-related stroke = 1.1%; device embolization = 0.4%).The rates of 7-day procedure-related adverse events in PREVAIL were generally substantially lower than PROTECT-AF but still higher than those in the LAAO Registry (pericardial effusion requiring surgery or pericardiocentesis = 1.9%; procedure-related stroke = 0.7%; device embolization = 0.7%). In EWOLUTION, the rate of 7-day procedure-related adverse events was 2.8%. The 1-day procedure-related adverse event rates reported in EWOLUTION were lower than the in-hospital adverse event rates we report from the LAAO Registry (major bleeding = 0.7%; pericardial effusion = 0.5%; device embolization = 0.2%).
Conclusions
The LAAO Registry is the largest registry of patients undergoing percutaneous LAAO procedures in the world. Hospital and physician procedural volumes vary substantially. The 38,000 patients enrolled in the LAAO Registry between 2016 and 2018 were at higher risk of both stroke and bleeding than those who participated in the clinical trials that led to FDA approval of the WATCHMAN device. However, despite this more complex patient population, implant success rates in contemporary practice were higher and in-hospital major adverse event rates were lower compared with those reported in the pivotal randomized trials. The LAAO Registry demonstrates the value of national registries to evaluate technologies as they are adopted in clinical practice and serves important roles for the clinical, scientific, and regulatory communities.
References
- Freeman JV, Varosy P, Price MJ, et al. The NCDR Left Atrial Appendage Occlusion Registry. J Am Coll Cardiol 2020;75:1503-18.
- Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018;137:e67-e492.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-51.
- Connolly SJ, Ezekowitz MD, Yusuf S, Reilly PA, Wallentin L. Newly identified events in the RE-LY trial. N Engl J Med 2010;363:1875-6.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-91.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093-104.
- Bartus K, Han FT, Bednarek J, et al. Percutaneous left atrial appendage suture ligation using the LARIAT device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol 2013;62:108-18.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 2019;16:e66-e93.
- Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009;374:534-42.
- Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1-12.
- Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA 2014;312:1988-98.
- Reddy VY, Doshi SK, Kar S, et al. 5-Year Outcomes After Left Atrial Appendage Closure: From the PREVAIL and PROTECT AF Trials. J Am Coll Cardiol 2017;70:2964-75.
- Lee RJ, Lakkireddy D, Mittal S, et al. Percutaneous alternative to the Maze procedure for the treatment of persistent or long-standing persistent atrial fibrillation (aMAZE trial): Rationale and design. Am Heart J 2015;170:1184-94.
- Lakkireddy D, Windecker S, Thaler D, et al. Rationale and design for AMPLATZER Amulet Left Atrial Appendage Occluder IDE randomized controlled trial (Amulet IDE Trial). Am Heart J 2019;211:45-53.
- Waksman R, Pendyala LK. Overview of the Food and Drug Administration circulatory system devices panel meetings on WATCHMAN left atrial appendage closure therapy. Am J Cardiol 2015;115:378-84.
- Decision Memo for Percutaneous Left Atrial Appendage (LAA) Closure Therapy (CAG-00445N) (Centers for Medicare &Medicaid Services website). February 2016. Available at: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=281. Accessed May 26, 2020.
- Masoudi FA, Ponirakis A, de Lemos JA, et al. Trends in U.S. Cardiovascular Care: 2016 Report From 4 ACC National Cardiovascular Data Registries. J Am Coll Cardiol 2017;69:1427-50.
- Messenger JC, Ho KK, Young CH, et al. The National Cardiovascular Data Registry (NCDR) Data Quality Brief: the NCDR Data Quality Program in 2012. J Am Coll Cardiol 2012;60:1484-8.
- Holmes DR Jr, Doshi SK, Kar S, et al. Left Atrial Appendage Closure as an Alternative to Warfarin for Stroke Prevention in Atrial Fibrillation: A Patient-Level Meta-Analysis. J Am Coll Cardiol 2015;65:2614-23.
- Phillips KP, Santoso T, Sanders P, et al. Left atrial appendage closure with WATCHMAN in Asian patients: 2 year outcomes from the WASP registry. Int J Cardiol Heart Vasc 2019;23:100358.
- Boersma LV, Schmidt B, Betts TR, et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: peri-procedural outcomes from the EWOLUTION registry. Eur Heart J 2016;37:2465-74.
- Reddy VY, Gibson DN, Kar S, et al. Post-Approval U.S. Experience With Left Atrial Appendage Closure for Stroke Prevention in Atrial Fibrillation. J Am Coll Cardiol 2017;69:253-61.
- Boersma LV, Ince H, Kische S, et al. Efficacy and safety of left atrial appendage closure with WATCHMAN in patients with or without contraindication to oral anticoagulation: 1-Year follow-up outcome data of the EWOLUTION trial. Heart Rhythm 2017;14:1302-8.
- Tzikas A, Holmes DR Jr, Gafoor S, et al. Percutaneous left atrial appendage occlusion: the Munich consensus document on definitions, endpoints, and data collection requirements for clinical studies. Europace 2017;19:4-15.
- Ledwoch J, Franke J, Gonzaga M, et al. Left atrial appendage closure: First in man with the 4th generation WATCHMAN device. Catheter Cardiovasc Interv 2016;87:787-94.
Clinical Topics: Anticoagulation Management, Arrhythmias and Clinical EP, Cardiac Surgery, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Pericardial Disease, Anticoagulation Management and Atrial Fibrillation, Implantable Devices, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Cardiac Surgery and Arrhythmias, Interventions and Imaging, Angiography, Nuclear Imaging
Keywords: Arrhythmias, Cardiac, Warfarin, Atrial Fibrillation, Stroke, Pericardiocentesis, Pericardial Effusion, Atrial Appendage, United States Food and Drug Administration, Coronary Angiography, Patient Selection, Follow-Up Studies, Prospective Studies, Bayes Theorem, Brain Ischemia, Medicaid, Medicare
< Back to Listings