Percutaneous Mitral Valve Therapies: The Old, Current, and Future
Management of chronic mitral regurgitation (MR) can be divided into medical or surgical/percutaneous. Choosing among these approaches depends on the etiology, severity, and patient comorbidities. Per the most recent American College of Cardiology expert consensus decision pathway on the management of MR, the principal treatment modality for primary MR is surgical repair with some role for transcatheter interventions, where medical and device therapies are the first option for treatment of secondary MR.1
Current guidelines give Class IIb recommendations for transcatheter mitral valve repair in symptomatic patients secondary to chronic severe primary MR despite optimal goal-directed medical therapy. Patients should have favorable anatomy and reasonable life expectancy but prohibitive surgical risk due to severe comorbidities.2 Because half of patients with primary severe MR are not surgical candidates due to older age, left ventricular (LV) dysfunction, and other comorbid conditions, the use of percutaneous mitral valve interventions has been increasing in the last few years.3
Compared to the aortic valve, mitral valve pathology is more complex, involving the valve leaflets and sometimes the annulus, making the transcatheter repair process more challenging. Percutaneous interventions can be divided into edge-to-edge leaflet repair, annuloplasty, mitral valve replacement, and other miscellaneous approaches. Currently, the only percutaneous technology approved by the US Food and Drug Administration (FDA) is the transcatheter mitral valve leaflet edge-to-edge percutaneous repair with the MitraClip device (Abbott Vascular; Abbott Park, IL).
In Europe, several devices have received the CE mark including MitraClip, PASCAL (Edwards Lifesciences; Irvine, CA), Mitralign (Mitralign, Inc.; Tewksbury, MA), Carillon (Cardiac Dimensions; Kirkland, WA), Cardioband (Edwards Lifesciences; Irvine, CA), NeoChord DS1000 (NeoChord, Inc.; St. Louis Park, MN), and, most recently, Tendyne (Abbott Vascular; Santa Clara, CA), which is the first transcatheter mitral valve implantation system to be approved in Europe. Multiple other repair techniques and devices are currently under investigation worldwide; these are listed in Table 1.
Table 1: Summary of Current Percutaneous Mitral Valve Repair Devices and Technique Currently in Evolution
| Edge-to-Edge Repair | Annuloplasty | Mitral Valve Replacement | Other Approaches |
| MitraClip* (Abbott Vascular; Abbott Park, IL) | Cardioband (Edwards Lifesciences; Irvine, CA) | Tendyne (Abbott Vascular; Santa Clara, CA) | NeoChord DS1000 (NeoChord, Inc.; St. Louis Park, MN) |
| PASCAL (Edwards Lifesciences; Irvine, CA) | Millipede IRIS (Millipede, Inc.; Santa Rosa, CA) | Tiara (Neovasc Inc; Richmond, B.C. Canada) | Harpoon TDS-5 (Edwards Lifesciences; Irvine, CA) |
| Valve clamp | Carillon (Cardiac Dimensions; Kirkland, WA) | EVOQUE (Edwards Lifesciences; Irvine, CA) | ChordArt (CoreMedic; Tübingen, Germany) |
| Mitral stitch | ARTO (MVRx, Inc.; San Mateo, CA) | Intrepid (Medtronic; Minneapolis, MN) | V-Chordal (Valtech Cardio Ltd.; Or-Yehuda, Israel) |
| AccuCinch (Ancora Heart; Santa Clara, CA) | Caisson (Caisson Interventional LLC; Maple Grove, MN) | Pipeline (Gore Medical; Newark, DE) | |
| Amend (Valcare Medical; Herzlyia Pituach, Israel) | HighLife (HighLife Medical, Inc.; Irvine CA) | CardioMech (CardioMech AS; Trondheim, Norway) | |
| Mitral cerclage | CardiAQ (Edwards Lifesciences; Irvine, CA) | Mitral Butterfly (Angel Valve Vienna; Vienna, Austria) | |
| MValve (Boston Scientific; Marlborough, MA) | |||
| SAPIEN M3 (Edwards Lifesciences; Irvine, CA) | |||
| NAVI (NaviGate Cardiac Structures Inc.; Lake Forest, CA) | |||
| AltaValve (4C Medical Technologies Inc.; Maple Grove, MN) | |||
| * Only MitraClip is FDA approved for use in the United States. | |||
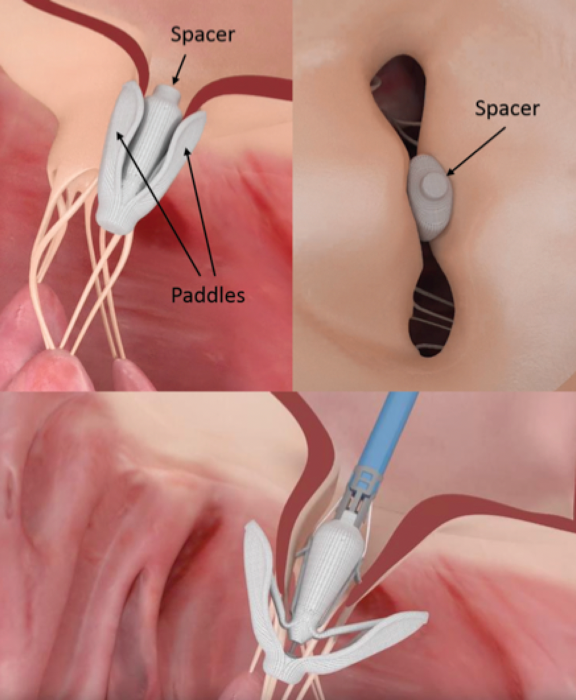
PASCAL Mitral Valve Repair System
The PASCAL mitral valve repair system uses an edge-to-edge repair technique with transseptal approach. The delivery system includes a 22-French guide with a steerable catheter and an implant catheter. Its implant consists of a central spacer that is designed to fill the regurgitant orifice area and two paddles and two clasps that allow for independent leaflet capture to optimize positioning (Figure 1). The device received its CE mark in February 2019.
Figure 1
The CLASP (The CLASP Study Edwards PASCAL TrAnScatheter Mitral Valve RePair System Study) study enrolled 62 patients with grade 3+ or 4+ MR. At 30 days, the PASCAL repair system showed feasibility and acceptable safety in the treatment of severe MR. This was accompanied by clinically and statistically significant improvements in functional status, exercise capacity, and quality of life.4 More recently, a 1-year follow-up of the CLASP study was published with analysis focused on functional and degenerative MR. A total of 109 patients was treated (67% functional MR, 33% degenerative MR), and the mean Society of Thoracic Surgeons score was 4.7%. The PASCAL transcatheter valve repair system demonstrated a low complication rate and high survival, with robust sustained MR reduction accompanied by significant improvements in functional status and quality of life at 1 year.5
Ongoing pivotal trials include CLASP IID/IIF (Edwards PASCAL CLASP IID/IIF Pivotal Clinical Trial), which is a prospective multicenter randomized controlled trial that aims to establish the safety and effectiveness of the PASCAL mitral repair system compared to MitraClip in patients with degenerative MR who have been determined to be at prohibitive risk for mitral valve surgery and patients with functional MR on guideline-directed medical therapy. The study is still in the recruiting phase; its estimated completion date is in January 2028.6 Another ongoing trial is the Edwards CLASP TR EFS trial, which is an early feasibility study to assess the safety and performance of the PASCAL system in patients with severe functional or degenerative tricuspid regurgitation who are symptomatic despite optimal medical therapy. It is currently recruiting in the United states with an estimated completion date in December 2025.7
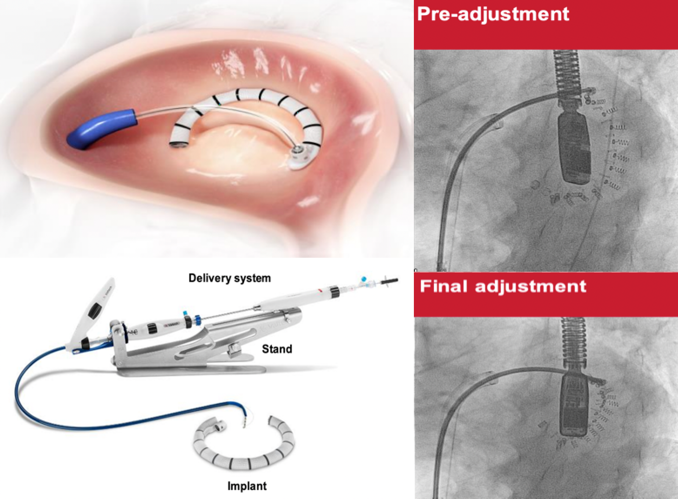
Cardioband Mitral Reconstruction System
The Cardioband system is a direct annuloplasty adjustable device that is implanted on the posterior mitral annulus under fluoroscopic and transesophageal echocardiographic guidance. The implant is made of a contraction wire with polyester fabric covering and is anchored into position by a series of stainless-steel anchors. The sequential anchors are later cinched through the wire, obtaining a controlled reduction in mitral valve annulus dimensions and reducing valvular regurgitation (Figure 2).
Figure 2
The Cardioband is available in 6 lengths to cover a wide range of annulus circumference sizes. It is implanted through a percutaneous transcatheter approach via a 24/25-French femoral venous sheath and transseptal puncture.8
The device received its CE mark in September 2015. It has not yet received FDA approval and therefore is not available for sale in the United States. The Cardioband system ACTIVE (Edwards Cardioband System ACTIVE Pivotal Clinical Trial) trial is currently underway to establish safety and effectiveness of the system in patients with functional MR and heart failure. It is estimated to enroll 375 participants with an approximate completion date in September 2024.9 Recently, Edwards Lifesciences has notified providers of a higher-than-expected rate of injuries during deployment of the Cardioband system. Approximately 5.7% of patients treated with Cardioband suffered coronary artery injuries, most as a result of direct interaction between the anchors of the device and the coronary arteries running adjacent to the valve annulus. The company said it will enhance existing warnings and instructions related to vessel injury avoidance, and all users will be trained or retrained on this potential issue and preventive measures before using Cardioband.
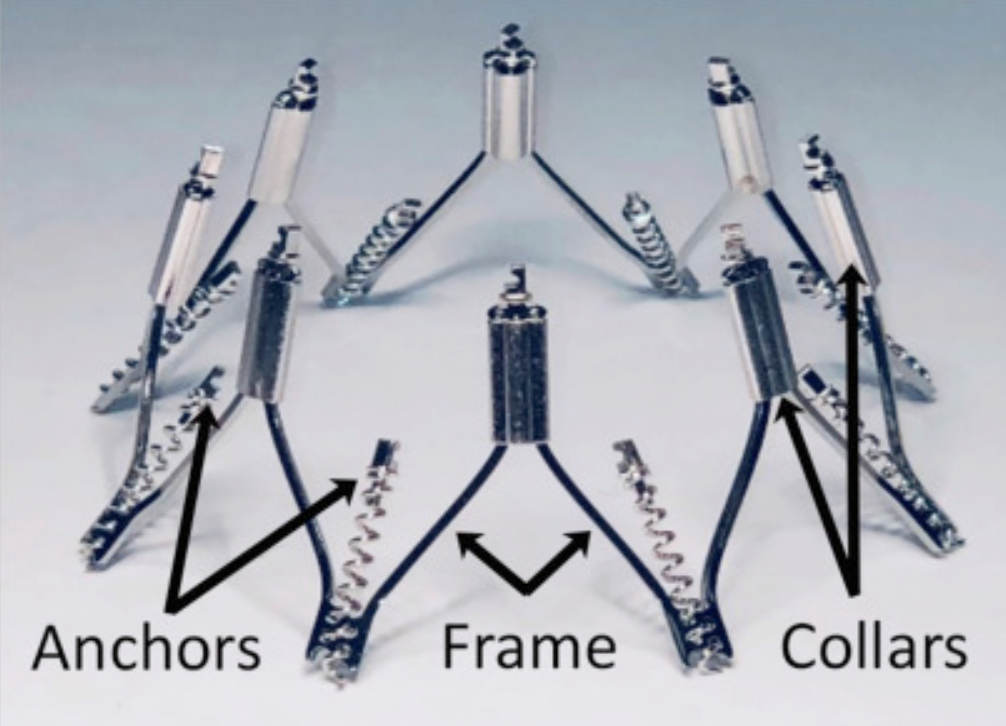
IRIS Complete Annuloplasty Ring
The IRIS device is a semi-rigid nitinol annuloplasty ring. It mimics surgical annuloplasty by reducing the mitral septal-lateral dimension and improving leaflet coaptation.
The ring consists of eight helical anchors pre-attached to the base of the device. Each anchor rotates independently and attaches directly to the mitral annulus and can be unscrewed and maneuvered to a different location if needed for optimal positioning. The upper portion of the device has eight sliding collars that can be tensioned, bringing the two adjacent anchors closer together (Figure 3). The IRIS system is implanted and manipulated on the atrial side, obviating concerns about LV chordal interaction. In the initial clinical report, there was no device-related procedural death, stroke, or myocardial infarction. Patients had significant reduction in mitral septolateral diameter along with significant improvement of MR and New York Heart Association class.10
Figure 3
In its most recent form, the IRIS system is implanted via a transfemoral venous transcatheter route through transseptal access to the left atrium, and it is adjustable, repositionable, and retrievable until customized to its final position and dimension. A trial to evaluate the IRIS system outside of the United States (NCT02607527) is underway, with estimated enrollment of 50 patients. The primary endpoint is acute safety, defined as incidence of adverse events, with a secondary endpoint of 30-day efficacy. This device has tricuspid valve repair applications as well.11
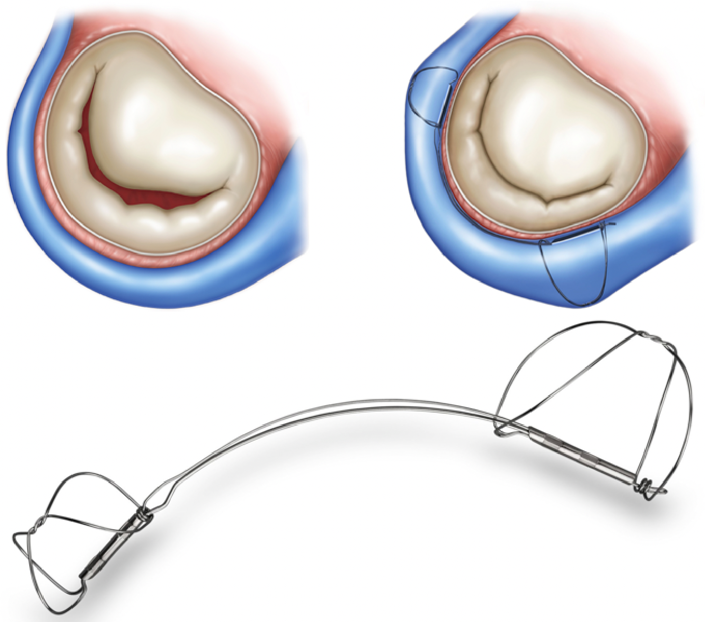
Carillon Mitral Contour System
The Carillon system is a right-heart transcatheter mitral annuloplasty device designed to reshape the anatomy and improve function of the mitral apparatus from the coronary sinus. The implantable device consists of a proximal anchor and a distal anchor connected by a shaping ribbon (Figure 4). It utilizes the heart's venous anatomy to cinch the mitral apparatus, reducing regurgitant volume without compromising the valve or future treatment options. The device can be recaptured and retrieved prior to release.13
Figure 4
Trials assessing the safety and efficacy of the Carillon device include TITAN and TITAN II (Transcatheter Implantation of Carillon Mitral Annuloplasty Device) and, most recently, the REDUCE FMR (CARILLON Mitral Contour System® for Reducing Functional Mitral Regurgitation) trial, which is the first sham-controlled double-blinded trial in valve therapy. It included 120 patients randomized into 2 groups (87 randomized to treatment and 33 randomized to sham control with guideline-directed medical therapy). The trial recently published its data showing that the Carillon device significantly reduced mitral regurgitant volume and LV volumes in symptomatic patients with functional MR receiving optimal medical therapy.13 Cardiac Dimensions has announced in a press release that its Carillon Mitral Contour System has now been implanted in 1,000 patients in the United States, Europe, Australia, Turkey, and the Middle East. It received its CE mark in September 2011. However, it is approved only for investigational use in the United States.
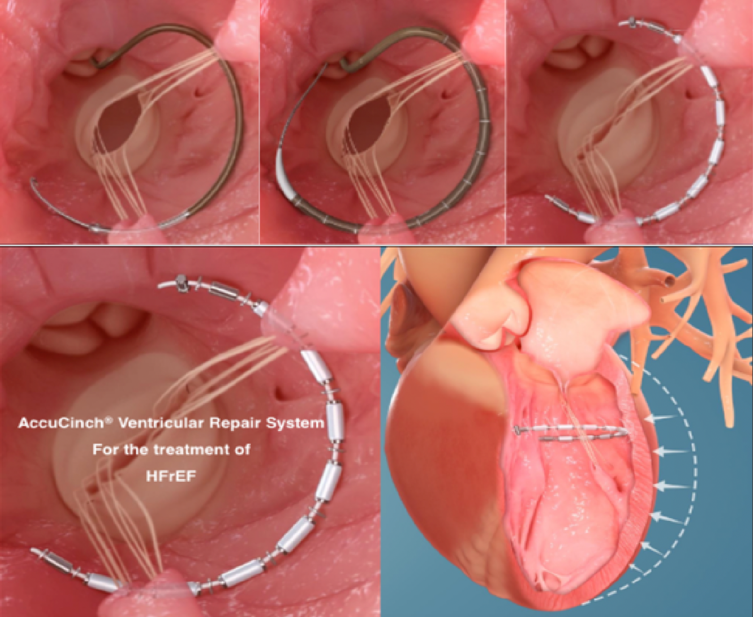
AccuCinch System
The AccuCinch system is designed to treat functional MR and LV remodeling by decreasing the circumference of the dilated LV, reducing its radius and reducing the mitral valve annulus dimensions. The device consists of anchors with self-expanding nitinol arms that fix the anchor into the myocardium. Anchors are positioned in the basal LV sub-annular space 10-20 mm below the mitral valve plane, and they are connected through a cable that is later cinched to bring the anchors together, reducing mitral annulus circumference (Figure 5). The system is delivered through a retrograde femoral arterial approach across the aortic valve.
Figure 5
CorCinch FMR (Early Feasibility Study of the AccuCinch® Ventricular Repair System), an early feasibility study of the AccuCinch system, is currently ongoing. Early data presented at 2019 Transcatheter Cardiovascular Therapeutics scientific meeting showed that the first 9 of 35 trial participants with heart failure and functional MR had an average 23% reduction in LV end systolic volume at 6 months. On average, ejection fraction improved from 31% to 39%, and scores on the Kansas City Cardiomyopathy Questionnaire increased by an average of 14%. The estimated study completion date is in March 2024. As of this writing, the device has not received a CE mark, nor is it FDA approved.14
The Amend Device
The Amend mitral annuloplasty ring is a semirigid, D-shaped ring that imitates surgical annuloplasty. The implantation procedure is done using a transapical surgical access, and the device is then advanced through a catheter across the mitral valve to the atrial aspect. When the catheter is in place, the ring gets released from the delivery catheter, changing its geometry and taking a D-shape configuration. It is then aligned with the mitral valve plane using a stabilizing tool to enable full control of the ring. Afterward, the ring is secured to the posterior mitral annulus via series of 12 independently deployed anchors. The posterior side of the annulus is first anchored and then pulled toward the anterior side, reducing the anteroposterior dimension by 15-25%. The anterior section then gets anchored to the anterior annulus, securing the device in place. This reduces mitral valve regurgitation and improves the leaflet coaptation (Figure 6).
Figure 6
The device can also serve as a landing zone for valve prosthesis if future mitral valve implantation is required. The device is still in the investigational phase. In November 2016, Valcare Medical announced its first-in-human experience, with successful device implantation in a patient at high surgical risk via transapical access at Sheba Medical Center in Israel. The AMEND I (AMENDTM Mitral Valve Repair System, Annuloplasty Ring Applied in a Transcatheter Method) study, which is a multi-center trial to evaluate the safety of the Amend mitral valve repair system, has completed its recruiting phase with an actual completion date in December 2018. However, no results have been reported so far.15
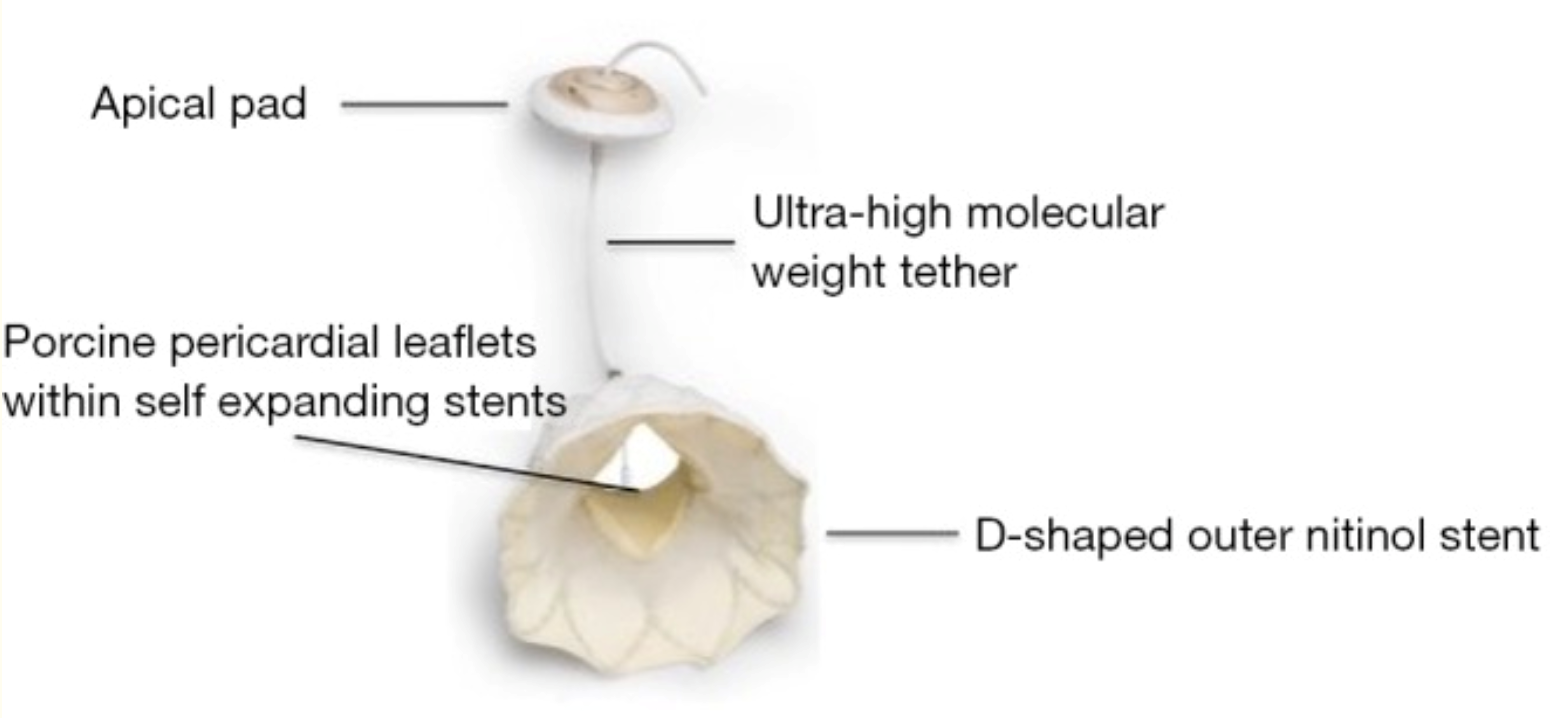
Tendyne System
The Tendyne system is a transapical transcatheter bioprosthetic mitral valve replacement device that is fully repositionable and retrievable. It consists of a porcine trileaflet valve fixed to a one-size inner stent that maintains an effective orifice area of at least 3.2 cm2. The outer stent is designed to match the D-shape of the native mitral annulus with 3 available sizes. Attached to the valve prosthesis is an ultra-high molecular weight apical tether that is secured to a pad resting on the apical epicardial surface (Figure 7). The system is delivered through a 34-French transapical sheath, accessed via a small left anterior thoracotomy.16 The device received the world's first CE mark for transcatheter mitral valve implantation in January 2020. An expanded clinical trial of the Tendyne mitral valve system (NCT02321514) is currently recruiting up to 350 subjects at up to 40 centers. Follow-up evaluations will be conducted through 5 years post-implantation. The estimated study completion date is in December 2024.17
Figure 7
NeoChord DS1000 System
The NeoChord DS1000 device is a minimally invasive mitral valve repair technology aimed at addressing degenerative MR. The device consists of a high-translucency expanded polytetrafluoroethylene synthetic fiber that is delivered via transapical access and is used as an artificial chord for mitral valve repair. After gaining access, the delivery system crosses the valve and grasps onto the affected leaflet, confirming the correct position by sending information from the jaws via fiber optic technology to a designated monitor. The delivery system then pierces the leaflet, delivering the cord and suturing it in place. The chord is thereby secured to the leaflet and then pulled and anchored to the myocardial apex site of entry (Figure 8). The whole process is done under echocardiographic guidance to ensure adequate chord length capable of achieving improvement in MR.
Figure 8
This device received its CE mark approval in 2012. In May 2016, NeoChord announced that it had received investigational device exemption approval from the FDA. ReChord (Randomized Trial of the Neochord DS1000 System Versus Open Surgical Repair) is the United States FDA pivotal study that started in November 2016, recruiting 585 patients with an aim to assess the safety and effectiveness of the device compared to open surgical repair. The study is estimated to be completed by July 2027.18
References
- O'Gara PT, Grayburn PA, Badhwar V, et al. 2017 ACC Expert Consensus Decision Pathway on the Management of Mitral Regurgitation: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2017;70:2421-49.
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017;70:252-89.
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J 2007;28:1358-65.
- Lim DS, Kar S, Spargias K, et al. Transcatheter Valve Repair for Patients With Mitral Regurgitation: 30-Day Results of the CLASP Study. JACC Cardiovasc Interv 2019;12:1369-78.
- Webb JG, Hensey M, Szerlip M, et al. One-Year Outcomes for Transcatheter Repair in Patients with Mitral Regurgitation from the CLASP Study. JACC Cardiovasc Interv 2020;June25:[Epub ahead of print].
- Edwards PASCAL CLASP IID/IIF Pivotal Clinical Trial (CLASP IID/IIF) (ClinicalTrials.gov website). June 5, 2020. Available at https://clinicaltrials.gov/ct2/show/NCT03706833. Accessed July 15, 2020.
- Edwards CLASP TR EFS (CLASP TR EFS) (ClinicalTrials.gov website). June 2, 2020. Available at https://clinicaltrials.gov/ct2/show/NCT03745313. Accessed July 15, 2020.
- Edwards Cardioband System ACTIVE Pivotal Clinical Trial (ACTIVE) (ACTIVE) (ClinicalTrials.gov website). June 11, 2020. Available at https://clinicaltrials.gov/ct2/show/NCT03016975. Accessed July 15, 2020.
- Miller M, Thourani VH, Whisenant B. The Cardioband transcatheter annular reduction system. Ann Cardiothorac Surg 2018;7:741-7.
- Rogers JH, Boyd WD, Smith TW, Bolling SF. Transcatheter Mitral Valve Direct Annuloplasty with the Millipede IRIS Ring. Interv Cardiol Clin 2019;8:261-7.
- Rogers JH, Boyd WD, Smith TW, Bolling SF. Early experience with Millipede IRIS transcatheter mitral annuloplasty. Ann Cardiothorac Surg 2018;7:780-6.
- Annular Reshaping of the Mitral Valve for Patients With Mitral Regurgitation Using the Millipede IRIS System (ClinicalTrials.gov website). March 30, 2020. Available at https://clinicaltrials.gov/ct2/show/NCT02607527. Accessed July 15, 2020.
- Witte KK, Lipiecki J, Siminiak T, et al. The REDUCE FMR Trial: A Randomized Sham-Controlled Study of Percutaneous Mitral Annuloplasty in Functional Mitral Regurgitation. JACC Heart Fail 2019;7:945-55.
- Shreenivas S. TCT 88: Six Month Outcomes of an Early Feasibility Study of the AccuCinch Left Ventricular Repair System in Patients with Heart Failure and Functional Mitral Regurgitation (TCTMD website). September 25, 2019. Available at https://www.tctmd.com/slide/tct-88-six-month-outcomes-early-feasibility-study-accucinch-left-ventricular-repair-system. Accessed July 15, 2020.
- AMENDTM Mitral Valve Repair System, Annuloplasty Ring Applied in a Transcatheter Method (ClinicalTrials.gov website). May 3, 2019. Available at https://clinicaltrials.gov/ct2/show/NCT02602613. Accessed July 15, 2020.
- Beller JP, Rogers JH, Thourani VH, Ailawadi G. Early clinical results with the Tendyne transcatheter mitral valve replacement system. Ann Cardiothorac Surg 2018;7:776-9.
- Expanded Clinical Study of the Tendyne Mitral Valve System (ClinicalTrials.gov website). July 7, 2020. Available at https://clinicaltrials.gov/ct2/show/NCT02321514. Accessed July 15, 2020.
- Randomized Trial of the Neochord DS1000 System Versus Open Surgical Repair (ReChord) (ClinicalTrials.gov website). December 5, 2019. Available at https://clinicaltrials.gov/ct2/show/NCT02803957. Accessed July 15, 2020.
Clinical Topics: Cardiac Surgery, Cardiovascular Care Team, Invasive Cardiovascular Angiography and Intervention, Valvular Heart Disease, Aortic Surgery, Cardiac Surgery and VHD, Interventions and Structural Heart Disease, Mitral Regurgitation
Keywords: Mitral Valve, Mitral Valve Annuloplasty, Mitral Valve Insufficiency, Aortic Valve, Coronary Sinus, Tricuspid Valve, Thoracotomy, Polytetrafluoroethylene, Feasibility Studies, Fiber Optic Technology, Radius, Incidence, Stainless Steel, Tricuspid Valve Insufficiency, Coronary Vessels, United States Food and Drug Administration, Life Expectancy, Quality of Life, Polyesters, Consensus, Prospective Studies
< Back to Listings