A Pediatric Perspective on the ACC/AHA Hypertrophic Cardiomyopathy Guidelines
Quick Takes
- Genetic testing with counseling should be offered to all patients with HCM and to first-degree relatives of genotype-positive patients to identify those genetically at-risk for developing HCM.
- All children, adolescents, and teenagers with HCM should undergo risk stratification for sudden cardiac death through the assessment of age-specific risk factors to help guide discussions surrounding primary prevention ICD for those at risk.
- Healthy recreational exercise should be encouraged to promote a heart healthy lifestyle since evidence supports that moderate (or even high intensity) exercise is safe; patients should be encouraged to participate in shared decision-making regarding exercise and ICD based on their preferences, lifestyle, and risk tolerance.
There have been impressive strides recently made in our understanding of hypertrophic cardiomyopathy (HCM). The 2020 American Heart Association / American College of Cardiology Guideline for the Diagnosis and Treatment of Patients with HCM provides important evidence and consensus-based guidelines to inform best clinical practices geared towards optimizing patient outcomes.1 For the first time, guidelines for the pediatric population are embedded within the main guidelines rather than as a separate consideration. This reflects a recognition that HCM is a familial disease that can manifest at any age in any family member including children. In fact, children are at high risk for developing major adverse cardiac events (including serious arrhythmic events) or to need major cardiac interventions within 2-3 years of diagnosis.2 The 2020 guidelines capture recent insights into the natural history of childhood HCM through several multicenter international cohort studies3-5 and ensure that this new evidence is incorporated into patient and physician decision making with a goal towards patient and family-centered care. Key messages of relevance to a pediatric population are summarized.
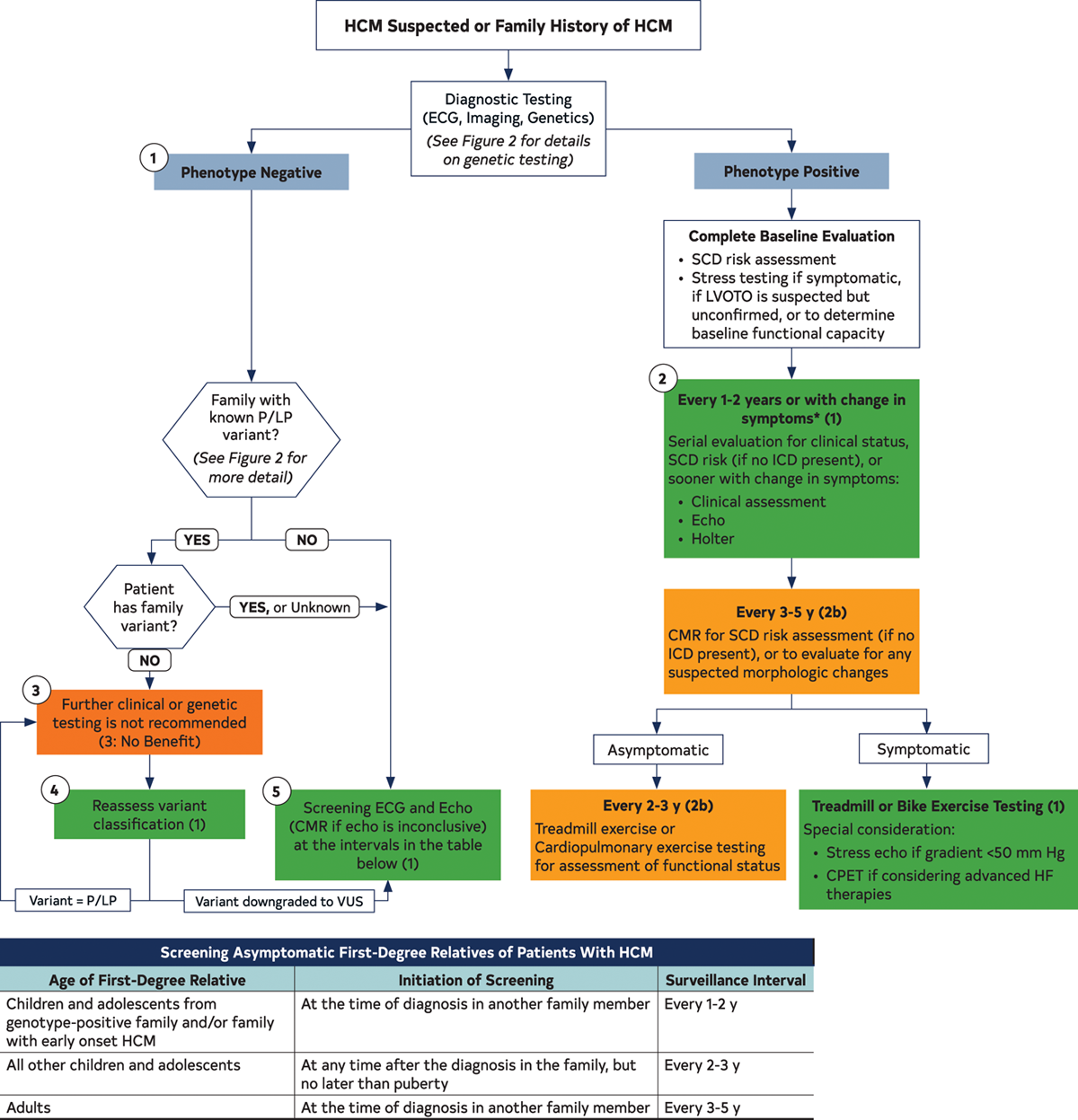
- Family screening: For any proband diagnosed with HCM, echocardiographic screening of first-degree relatives should be initiated independent of their age. Surveillance clinical echocardiographic screening should continue every 1-3 years since HCM can manifest at any age within the family.2
- Genetic screening: Genetic testing with counseling should be offered to all patients with HCM.6 For HCM patients who have pathogenic variants on genetic testing, cascade genetic testing should be offered to all first-degree relatives with ongoing clinical surveillance indicated only in those relatives who carry the pathogenic variant. For HCM patients who have no pathogenic variants on genetic testing, cascade genetic testing of the family is not useful. It is important to reassess variant pathogenicity every 2-3 years as variant classification may evolve with time and alter the recommendations for ongoing family screening.7 Figure 1 shows the approach to genetic screening.
Figure 1: Recommended Evaluation and Testing for HCM
- Cardiac imaging: While echocardiography remains the cornerstone in the diagnosis of HCM, cardiovascular magnetic resonance (CMR) imaging can be helpful in patients in whom there is diagnostic uncertainty e.g., suspected metabolic storage disorder, poor echocardiographic imaging windows, or as an adjunct in sudden cardiac death (SCD) risk assessment through measurement of late gadolinium enhancement.8
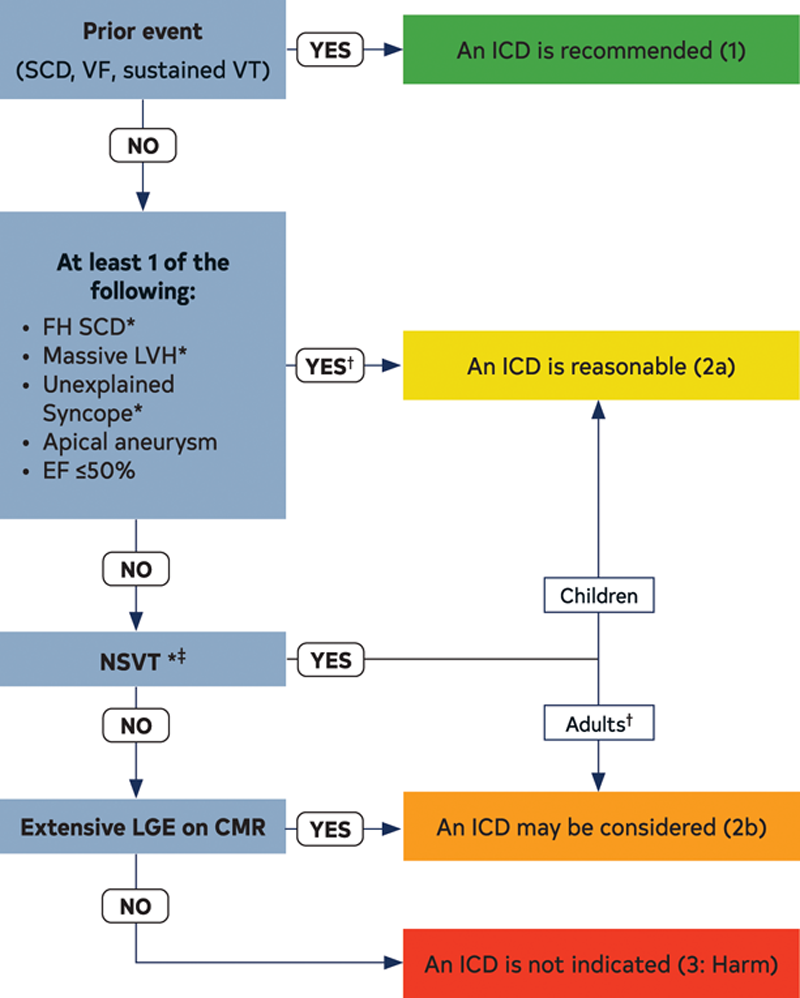
- SCD risk assessment: SCD risk stratification in children should incorporate pediatric-specific risk factors, since many risk factors in children carry different weights than those in adults.3,4,9 For example, unlike adults, in children there is a non-linear association of left ventricular (LV) hypertrophy with SCD risk; therefore there is no absolute threshold septal diameter z score that can be used as a cut-off to recommend an implantable cardioverter defibrillator (ICD) for primary prevention.3,4 LV outflow tract gradient and family history of SCD are not associated with higher SCD risk in children the same way as in adults, although the latter may reflect a lag between diagnosis and emergence of SCD events in young adult family members. Figure 2 shows the approach to SCD risk assessment in children and adults.
Figure 2: ICD Patient Selection
- ICD indications: ICD is recommended for secondary prevention in any child with HCM with a previous documented cardiac arrest or sustained ventricular tachycardia. ICD placement for primary prevention is reasonable for children with HCM who have conventional risk factors including unexplained syncope, massive LV hypertrophy, non-sustained ventricular tachycardia, or family history of early HCM-related SCD (Figure 2). However, ICD implantation for primary prevention in young children often requires a higher threshold than in adults (e.g., the presence of more than one risk factor) given the complexity of ICD placement as well as a higher risk of device complications in young children.10 In patients with unclear risk stratification, extensive late gadolinium enhancement on CMR imaging may complement risk stratification.
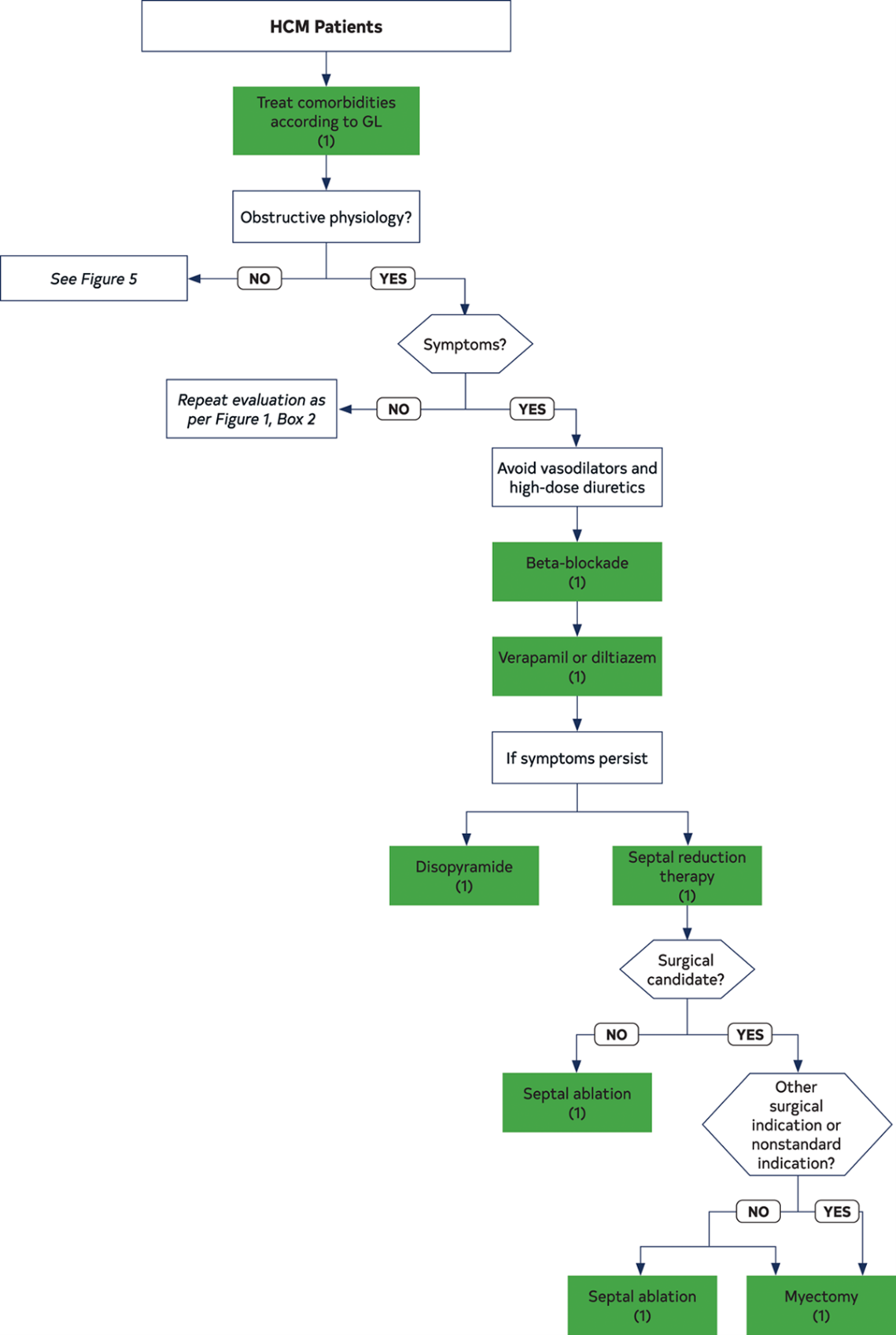
- Treatment for LV outflow tract obstruction: Septal reduction therapy is indicated in symptomatic, drug-refractory patients with persistent resting or provocable LV outflow tract gradient >50mmHg, with surgical myectomy being the recommended approach in children (Figure 3). Surgery should be performed at HCM centers with experienced teams.11
Figure 3: Management of Symptoms in Patients with HCM
- Shared decision making: It is important to understand a patient and family preferences regarding ICD, exercise, genetic testing, family screening, and type and timing of interventions. The physician should engage the patient and family in a shared decision-making process that incorporates patient's lifestyle choices and preferences when making complex, potentially life-altering decisions.12
- Exercise: A heart healthy lifestyle should be promoted in all patients and this includes avoiding unnecessary exercise restrictions. There is growing evidence that healthy recreational exercise (moderate intensity) has not been associated with increased risk of ventricular arrhythmias.13-15 It is reasonable for genotype-positive, phenotype-negative patients to participate in higher intensity competitive athletics,16 and competitive level participation may be acceptable in select phenotype-positive patients after full disclosure and discussion of risks and benefits. ICD placement for the sole purpose of participation in competitive athletics should not be performed.
- Multi-disciplinary care model: HCM management needs to evolve as disease penetrance, severity and manifestations evolve with age.17 This requires that the patient and family have access to a multi-disciplinary team that includes cardiologists with cardiomyopathy expertise, electrophysiologists, experts in echocardiography and CMR imaging, cardiothoracic surgeons, interventional cardiologists, geneticists, and genetic counselors. Referral to multidisciplinary HCM centers with graduated levels of expertise is recommended for complex decision making and advanced therapies in the HCM population.
The 2020 guidelines also identify the need for future research in areas with unmet needs in order to inform continual evidence-driven updates to the guidelines. These areas include precision medicine approaches, wearables for physiological monitoring for arrhythmias, shared decision-making supports for physicians and patients, efforts to increase genetic diagnoses and genetic awareness, integration of genotype information into risk prediction models, as well as clinical trials of genotype-guided and targeted therapies.
Table 1
References
- Ommen SR, Mital S, Burke MA, et al. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2020;76:3022-55.
- Lafreniere-Roula M, Bolkier Y, Zahavich L, et al. Family screening for hypertrophic cardiomyopathy: is it time to change practice guidelines? Eur Heart J 2019;40:3672-81.
- Balaji S, DiLorenzo MP, Fish FA, et al. Risk factors for lethal arrhythmic events in children and adolescents with hypertrophic cardiomyopathy and an implantable defibrillator: an international multicenter study. Heart Rhythm 2019;16:1462-67.
- Miron A, Lafreniere-Roula M, Steve Fan CP, et al. A validated model for sudden cardiac death risk prediction in pediatric hypertrophic cardiomyopathy. Circulation 2020;142:217-29.
- Norrish G, Ding T, Field E, et al. Development of a novel risk prediction model for sudden cardiac death in childhood hypertrophic cardiomyopathy (HCM Risk-Kids). JAMA Cardiol 2019;4:918-27.
- Ahmad F, McNally EM, Ackerman MJ, et al. Establishment of specialized clinical cardiovascular genetics programs: recognizing the need and meeting standards: a scientific statement from the American Heart Association. Circ Geonom Precis Med 2019;12:e000054.
- Deignan JL, Chung WK, Kearney HM, Monaghan KG, Rehder CW, Chao EC. Points to consider in the reevaluation and reanalysis of genomic test results: a statement of the American College of Medical Genetics and Genomics (ACMG). Genet Med 2019;21:1267-70.
- Weng Z, Yao J, Chan RH, et al. Prognostic value of LGE-CMR in HCM: a meta-analysis. JACC Cardiovasc Imaging 2016;9:1392-1402.
- Norrish G, Cantarutti N, Pissaridou E, et al. Risk factors for sudden cardiac death in childhood hypertrophic cardiomyopathy: a systematic review and meta-analysis. Eur J Prev Cardiol 2017;24:1220-30.
- Krause U, Müller MJ, Wilberg Y, et al. Transvenous and non-transvenous implantable cardioverter-defibrillators in children, adolescents, and adults with congenital heart disease: who is at risk for appropriate and inappropriate shocks? Europace 2019;21:106-13.
- Arghami A, Dearani JA, Said SM, O'Leary PW, Schaff HV. Hypertrophic cardiomyopathy in children. Ann Cardiothorac Surg 2017;6:376-85.
- Légaré F, Adekpedjou R, Stacey D, et al. Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst Rev 2018;7:Cd006732.
- Lampert R, Olshansky B, Heidbuchel H, et al. Safety of sports for athletes with implantable cardioverter-defibrillators: long-term results of a prospective multinational registry. Circulation 2017;135:2310-12.
- Pelliccia A, Lemme E, Maestrini V, et al. Does sport participation worsen the clinical course of hypertrophic cardiomyopathy? Clinical outcome of hypertrophic cardiomyopathy in athletes. Circulation 2018;137:531-33.
- Saberi S, Wheeler M, Bragg-Gresham J, et al. Effect of moderate-intensity exercise training on peak oxygen consumption in patients with hypertrophic cardiomyopathy: a randomized clinical trial. JAMA 2017;317:1349-57.
- Maurizi N, Michels M, Rowin EJ, et al. Clinical course and significance of hypertrophic cardiomyopathy without left ventricular hypertrophy. Circulation 2019;139:830-33.
- Ho CY, Day SM, Ashley EA, et al. Genotype and lifetime burden of disease in hypertrophic cardiomyopathy: insights from the Sarcomeric Human Cardiomyopathy Registry (SHaRe). Circulation 2018;138:1387-98.
Clinical Topics: Arrhythmias and Clinical EP, Congenital Heart Disease and Pediatric Cardiology, Heart Failure and Cardiomyopathies, Noninvasive Imaging, Prevention, Sports and Exercise Cardiology, Implantable Devices, SCD/Ventricular Arrhythmias, Atrial Fibrillation/Supraventricular Arrhythmias, Congenital Heart Disease, CHD and Pediatrics and Arrhythmias, CHD and Pediatrics and Imaging, CHD and Pediatrics and Prevention, CHD and Pediatrics and Quality Improvement, Acute Heart Failure, Echocardiography/Ultrasound, Sports and Exercise and Congenital Heart Disease and Pediatric Cardiology, Sports and Exercise and Imaging
Keywords: Heart Failure, Pediatrics, Heart Defects, Congenital, Gadolinium, Contrast Media, Defibrillators, Implantable, Secondary Prevention, American Heart Association, Patient Selection, Decision Making, Cardiomyopathy, Hypertrophic, Arrhythmias, Cardiac, Echocardiography, Primary Prevention, Genetic Testing, Risk Assessment, Tachycardia, Ventricular, Risk Factors, Syncope, Sports, Monitoring, Physiologic, Hypertrophy, Genotype, Magnetic Resonance Spectroscopy, Patient-Centered Care, Pharmaceutical Preparations
< Back to Listings