Diastolic Risk Markers
Quick Takes
- Current guidelines for assessing diastolic dysfunction are problematic to apply in routine clinical practice.
- A "mortality threshold" has emerged that may help identify those at increased long-term mortality risk irrespective of the underlying cause for diastolic dysfunction.
- The inclusion of age in the interpretation of diastolic function may enhance the prognostic significance of certain abnormal measures.
Diastolic filling of the left ventricle (LV) is a highly complex process that is dependent on LV relaxation, LV compliance, and left atrial pressure. Impaired LV relaxation and compliance results in subsequent increases in left atrial pressure and eventual heart failure. Importantly, abnormal diastolic function impairs exercise capacity1 and quality of life. Moreover, in specific patient groups such as heart failure2,3 and post-myocardial infarction,4 abnormal diastolic function is associated with increased mortality.5 For these reasons, routine measurement of diastolic function during echocardiography is recommended in international guidelines.6 However, several challenges remain. These include 20-50% of patients being classified as having "indeterminate" diastolic function if guideline algorithms are applied.7,8 Furthermore, normal age-related changes in diastolic function are not routinely considered.
Multiple cardiac disease states may impair diastolic function, with important disease-specific differences. Similarly, some cardiac interventions may adversely affect some diastolic measurements more than others. These include cardiac surgery, aortic or mitral valve intervention, left bundle branch block, and ventricular pacing. Despite the myriad conditions that may adversely affect LV diastolic function in disease-specific ways, a key clinical goal is to correctly identify high-risk individuals. To explore this, our group undertook two important, interrelated analyses using NEDA (National Echocardiography Database of Australia), a large observational database that captures routinely acquired echocardiographic data on a retrospective and prospective basis in Australia.9 Individual data linkage is then used to derive health outcomes, including mortality.10 These analyses were presented at the European Society of Cardiology Congress 2020 and published in the European Heart Journal: Cardiovascular Imaging.11
The first NEDA-based analysis examined the profile and prognostic impact of guideline-derived diastolic function assessment across the broad patient population managed in routine clinical practice. The second analysis examined the pattern of mortality associated with each of the main diastolic parameters measured on echocardiography. Overall, 436,360 individuals with at least 1 valid diastolic measurement formed the study cohort.9 American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) guideline-derived categories of diastolic dysfunction could be applied in 392,009 cases (90%). We demonstrated that abnormal diastolic function, defined by ASE/EACVI diastolic function criteria is, as expected, associated with increased risk of cardiovascular-related and all-cause mortality. However, consistent with other reports,7,8,12 indeterminate diastolic function using the ESC/EACVI algorithm was common (comprising 21.5% of individuals overall and 62.2% of those with reduced LV ejection fraction). These data highlight a typical clinical dilemma when the guideline recommendations are applied in isolation.
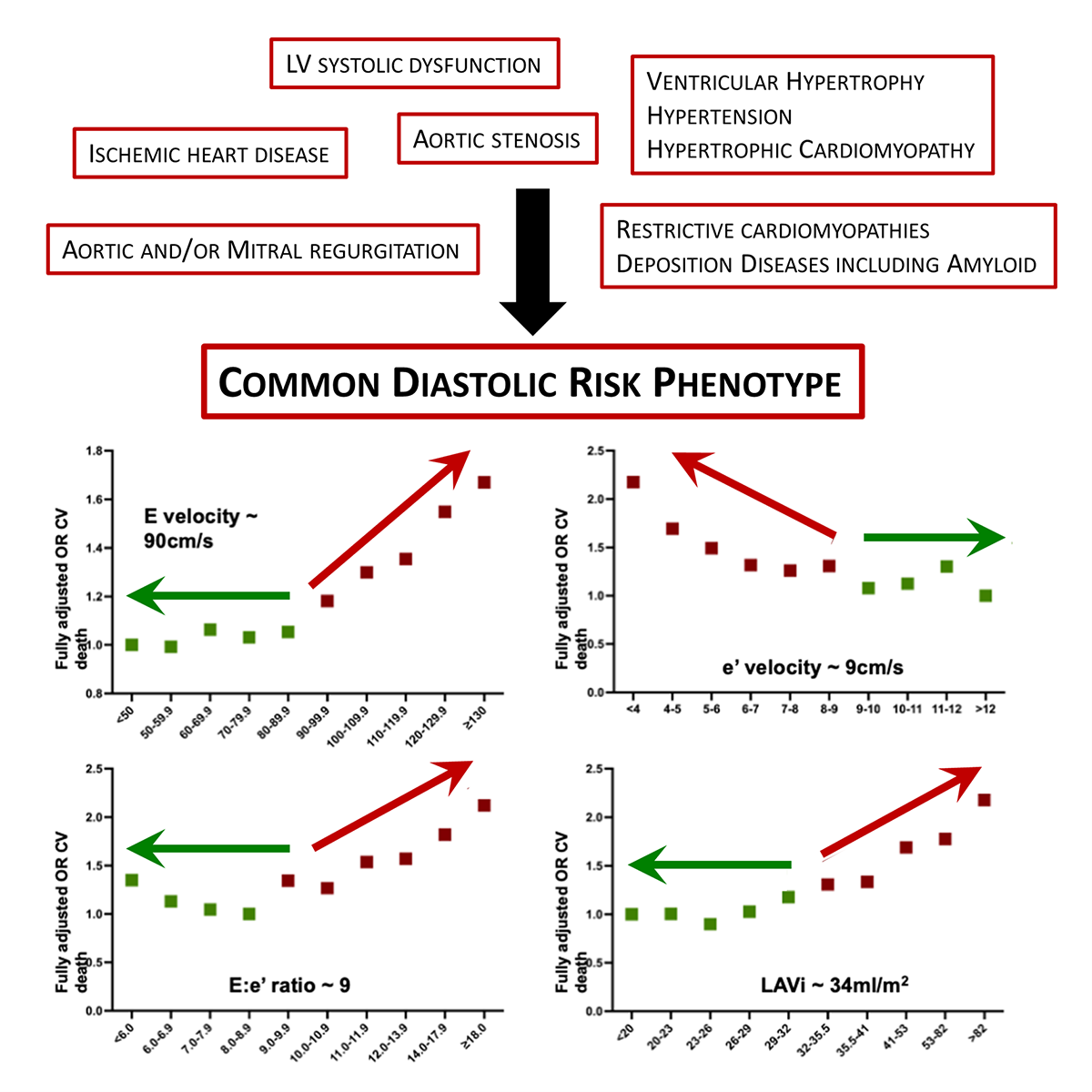
Our subsequent analyses of the prognostic significance of the four most-informative diastolic function parameters were more encouraging. Specifically, we focused on mitral E wave velocity (E velocity), septal myocardial e' velocity (e' velocity), the septal E:e' ratio (E:e' ratio), and the left atrial volume index (LAVi). After adjustments for age, sex, LV ejection fraction, rhythm (including presence of atrial fibrillation), valvular heart disease, and mitral or aortic valve replacement, there was a clear pivot point where mortality increased. A simple "rule of 9s" pattern of mortality emerged from the data, where mortality increased above 90 cm/s for E velocity, below 9 cm/s for e' velocity, above 9 for E:e' ratio, and above 34 ml/m2 for LAVi (Figure 1). Increasing age significantly affected each parameter, as did the cardiac rhythm (such as the presence of atrial fibrillation), but we did not observe any important sex-based differences.
Figure 1: Common Diastolic Risk Phenotype
In summary, current guidelines for assessing diastolic dysfunction are problematic to apply in routine clinical practice. Alternatively, irrespective of the underlying cause for diastolic dysfunction, which requires rigorous investigation for each patient, it appears that a "mortality threshold" has emerged that may be helpful in identifying those at increased long-term mortality risk, shown in Figure 1. There are important questions that arise from our analyses. It is not yet clear whether crossing the "rule of 9s" threshold is of clinical importance if just one variable is affected or whether each parameter is of equal importance. For example, an E velocity above 90 cm/s may occur in a healthy young person or as a physiological response to volume loading.13 However, in this situation, the e' velocity would be expected to be preserved. Similarly, an e' velocity <9 cm/s may occur in healthy older individuals in the absence of other diastolic abnormalities. The inclusion of age in the interpretation of diastolic function may enhance the prognostic significance of certain abnormal measures and warrants further research and consideration in future guidelines.
Taken together, these data suggest a phenotypic response to a broad range of cardiac diseases that may result in diastolic abnormalities and may be useful in interpretation of routine clinical echocardiography, particularly when a guideline-based appraisal is unable to assist in assessing an individual's risk of premature mortality.
References
- Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure With Preserved Ejection Fraction. Circulation 2018;138:861-70.
- Rossi A, Temporelli PL, Quintana M, et al. Independent relationship of left atrial size and mortality in patients with heart failure: an individual patient meta-analysis of longitudinal data (MeRGE Heart Failure). Eur J Heart Fail 2009;11:929-36.
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355:251-9.
- Møller JE, Whalley GA, Dini FL, et al. Independent prognostic importance of a restrictive left ventricular filling pattern after myocardial infarction: an individual patient meta-analysis: Meta-Analysis Research Group in Echocardiography acute myocardial infarction. Circulation 2008;117:2591-8.
- Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation 2011;123:2006-13.
- Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277-314.
- Gottbrecht MF, Salerno M, Aurigemma GP. Evolution of diastolic function algorithms: Implications for clinical practice. Echocardiography 2018;35:39-46.
- Luke P, Eggett C, Spyridopoulos I, Irvine T. A comparative analysis of British and American Society of Echocardiography recommendations for the assessment of left ventricular diastolic function. Echo Res Pract 2018;5:139-47.
- Strange G, Celermajer DS, Marwick T, et al. The National Echocardiography Database Australia (NEDA): Rationale and methodology. Am Heart J 2018;204:186-9.
- Strange G, Stewart S, Celermajer D, et al. Poor Long-Term Survival in Patients With Moderate Aortic Stenosis. J Am Coll Cardiol 2019;74:1851-63.
- Playford D, Strange G, Celermajer DS, et al. Diastolic dysfunction and mortality in 436 360 men and women: the National Echo Database Australia (NEDA). Eur Heart J Cardiovasc Imaging 2021;22:505-15.
- Almeida JG, Fontes-Carvalho R, Sampaio F, et al. Impact of the 2016 ASE/EACVI recommendations on the prevalence of diastolic dysfunction in the general population. Eur Heart J Cardiovasc Imaging 2018;19:380-6.
- Caballero L, Kou S, Dulgheru R, et al. Echocardiographic reference ranges for normal cardiac Doppler data: results from the NORRE Study. Eur Heart J Cardiovasc Imaging 2015;16:1031-41.
Clinical Topics: Arrhythmias and Clinical EP, Cardiac Surgery, Heart Failure and Cardiomyopathies, Invasive Cardiovascular Angiography and Intervention, Noninvasive Imaging, Valvular Heart Disease, EP Basic Science, Atrial Fibrillation/Supraventricular Arrhythmias, Aortic Surgery, Cardiac Surgery and Arrhythmias, Cardiac Surgery and Heart Failure, Cardiac Surgery and VHD, Acute Heart Failure, Interventions and Imaging, Interventions and Structural Heart Disease, Echocardiography/Ultrasound
Keywords: Diagnostic Imaging, Quality of Life, Heart Ventricles, Mitral Valve, Aortic Valve, Bundle-Branch Block, Atrial Fibrillation, Atrial Pressure, Retrospective Studies, Mortality, Premature, Stroke Volume, Prospective Studies, Echocardiography, Heart Failure, Myocardial Infarction, Heart Valve Diseases, Cardiac Surgical Procedures, Outcome Assessment, Health Care, Heart Atria
< Back to Listings